Abstract
Myrrhanone C [8(R)-3-oxo-8-hydroxypolypoda-13E,17E,21-triene], a bicyclic triterpene isolated from the gum resin of Commiphora mukul, has been chemically transformed to synthesize a series of ten novel pyrimidine hybrids in good to excellent yields. The synthesized compounds (2–22) were evaluated for their anticancer potential against a panel of six cancer cell lines, namely A-549 (lung), Hela (cervical), MCF-7 (breast), ACHN (renal), Colo-205 (colon) and B-16 (mouse melanoma) by employing the MTT assay. In general, the synthesized compounds showed significant anticancer activity against all the cancer cell lines tested. Interestingly, the pyrimidine hybrids 18 and 19 showed good activity against the A-549, MCF-7, B-16, Colo-205 and ACHN cancer cell lines with \(\hbox {IC}_{50}\) values between 7.7–37.8 \(\upmu \)M. Most significantly, compounds 19 (IC\(_{50}\): 7.7 \(\upmu \)M) and 18 (IC\(_{50}\): 9.5 \(\upmu \)M) showed about five- and six-fold enhanced activities, respectively, compared to the parent myrrhanone C (1) against A-549 cell line. Flow cytometric analysis revealed that compounds 18 and 19 induced apoptosis in A-549 cells and arrested the cell growth in the G0/G1 phase.
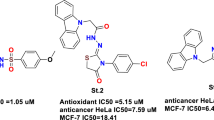
Graphical Abstract






Similar content being viewed by others

References
Daniel AD, Sylvia U, Ute R (2012) A historical overview of natural products in drug discovery. Metabolites 2:303–336. doi:10.3390/metabo2020303
Guo Z (2012) Modification of natural products for drug discovery. Yao Xue Xue Bao 47:144–157
Yadav VR, Prasad S, Sung Ramaswamy BK, Aggarwal BB (2010) Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2:2428–2466. doi:10.3390/toxins2102428
Hanus LO, Rezanka T, Dembitsky VM, Moussaieff A (2005) Myrrh-commiphora chemistry. Biomed Papers 149:3–28. doi:10.5507/bp.2005.001
Matsuda H, Morikawa T, Ando S, Oominami H, Murakami T, Kimura I, Yoshikawa M (2004) Absolute stereostructures of polypodane-type triterpenes, myrrhanol A and myrrhanone A, from guggul-gum resin (the resin of Balsamodendron mukul). Chem Pharm Bull 52:1200–1203. doi:10.1248/cpb.52.1200
Matsuda H, Morikawa T, Ando S, Oominami H, Murakami T, Kimura I, Yoshikawa M (2004) Absolute stereostructures of polypodane- and octanordammarane-type triterpenes with nitric oxide production inhibitory activity from guggul-gum resins. Bioorg Med Chem 12:3037–3046. doi:10.1016/j.bmc.2004.03.020
Samy Morad AF, Claudia S, Berthold B, Bernd S, Michael W, Tatiana S, Thomas S (2011) (8R)-\(3\beta \),8-Dihydroxypolypoda-13E,17E,21-triene induces cell cycle arrest and apoptosis in treatment-resistant prostate cancer cells. J Nat Prod 74:1731–1736. doi:10.1021/np200161a
Victoriano D, Lidia L, José F, Alejandro F (2013) First synthesis of (\(+\))-myrrhanol C, an anti-prostate cancer lead. Org Biomol Chem 11:559–562. doi:10.1039/c2ob26947c
Amr AEGE, Sabry NM, Abdulla MM (2007) Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatshefte für Chemie 138:699–707. doi:10.1007/s00706-007-0651-0
Fujiwara N, Nakajima T, Ueda Y, Fujita H, Kawakami H (2008) Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg Med Chem 16:9804–9816. doi:10.1016/j.bmc.2008.09.059
Wagner E, Al-Kadasi K, Zimecki M, Sawka-Dobrowolska W (2008) Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur J Med Chem 43:2498–2504. doi:10.1016/j.ejmech.2008.01.035
Mallavadhani UV, Mahapatra A, Pattnaik B, Vanga NR, Suri N, Saxena KA (2013) Synthesis and anti-cancer activity of some novel C-17 analogs of ursolic and oleanolic acids. Med Chem Res 22:1263–1269. doi:10.1007/s00044-012-0106-y
Mallavadhani UV, Vanga NR, Jeengar MK, Naidu VGM (2014) Synthesis of novel ring-A fused hybrids of oleanolic acid with capabilities to arrest cell cycle and induce apoptosis in breast cancer cells. Eur J Med Chem 74:398–404. doi:10.1016/j.ejmech.2013.12.040
Selvam TP, Karthick V, Kumar PV, Ali MA (2012) Synthesis and structure–activity relationship study of 2-(substitutedbenzylidene)-7-(4-fluorophenyl)-5-(furan-2-yl)-\(2H\)-thiazolo[3,2-\(a\)]pyrimidin-3(\(7H\))-one derivatives as anticancer agents. Drug Discov Ther 6:198–204. doi:10.5582/ddt.2012.v6.4.198
EI-Rayyes NR, Ramadan HM (1987) Synthesis of new benzo[6,7]cyclohepta[1,2-d] pyrimidine. J Heterocycl Chem 24:1141–1147. doi:10.1002/jhet.5570240442
Ahluwalia VK, Kaila N, Bala S (1987) Synthesis & antifungal & antibacterial activities of some 2-amino-4,6-substituted-pyrimidines & 4-styryl-6,7-pyranocoumarins. Indian J Chem Sec B 26B:700–702
Pasha TY, Udipi RH, Bhat AR (2005) Synthesis and antimicrobial screening of some pyrimidine derivatives. Indian J Heterocycl Chem 15:149–152
Botta M, Armaroli S, Castagnolo D, Fontana G, Perad P, Bombardelli E (2007) Synthesis and biological evaluation of new taxoids derived from 2-deacetoxytaxinine. Bioorg Med Chem Lett 17:1579–1583. doi:10.1016/j.bmcl.2006.12.101
Sun SY, Hail N Jr, Lotan RJ (2004) Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst 96:662–672. doi:10.1093/jnci/djh123
Hotz MA, Gong J, Traganos F, Darzynkiewicz Z (1994) Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry 15:237–244. doi:10.1002/cyto.990150309
Konstantinov SM, Berger MR (1999) Human urinary bladder carcinoma cell lines respond to treatment with alkylphosphocholines. Cancer Lett 144:153–160. doi:10.1016/S0304-3835(99)00219-0
Henkels PM, Turchi JJ (1999) Cisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell lines. Cancer Res 59:3077–3083
Francis JA, Raja SN, Nair MG (2004) Bioactive terpenoids and guggulusteroids from Commiphora mukul gum resin of potential anti-inflammatory interest. Chem Biodiver 1:1842–1853. doi:10.1002/cbdv.200490138
Kamal A, Ramakrishna G, Lakshma Nayak V, Raju P, Subba Rao AV, Viswanath A, Vishnuvardhan MVPS, Ramakrishna S, Srinivas G (2012) Design and synthesis of benzo[c, d] indolone–pyrrolobenzodiazepine conjugates as potential anticancer agents. Bioorg Med Chem 20:789–800. doi:10.1016/j.bmc.2011.12.003
Cosse JP, Sermeus A, Vannuvel K, Ninane N, Raes M, Michiels C (2007) Differential effects of hypoxia on etoposide-induced apoptosis according to the cancer cell lines. Mol Cancer 6:1–16. doi:10.1186/1476-4598-6-61
Acknowledgments
We are thankful to Director, CSIR-IICT for support and encouragement. We are also thankful to CSIR, India for providing fellowship to MC and DBT, Govt. of India for partial funding under the co-ordinated project “Biotechnological Interventions for Pharmaceutically Valuable Compounds from Forest Resins” (GAP-0412).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mallavadhani, U.V., Chandrashekhar, M., Nayak, V.L. et al. Synthesis and anticancer activity of novel fused pyrimidine hybrids of myrrhanone C, a bicyclic triterpene of Commiphora mukul gum resin. Mol Divers 19, 745–757 (2015). https://doi.org/10.1007/s11030-015-9621-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9621-3