Abstract
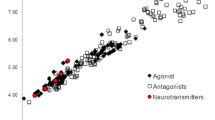
Extensive biochemical and clinical studies have increasingly recognized Parkinson’s disease as a highly complex and multi-faceted neurological disorder having branched non-motor symptoms including sleep disorders, pain, constipation, psychosis, depression, and fatigue. A wide range of biological targets in the brain deeply implicated in this pathology resulted in a plethora of novel small-molecule compounds with promising activity. This review thoroughly describes the chemical space of non-dopamine receptor ligands in terms of diversity, isosteric/bioisosteric morphing, and molecular descriptors.












Similar content being viewed by others
References
Pahwa E, Lyons KE (2007) Handbook of Parkinson’s disease, 4th edn., Neurological disease and therapyCRC Press, Boca Raton
Grayson M (2010) Parkinson’s disease. Nature 466:S1. doi:10.1038/466S2a
Kapetanovic IM (2008) Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem Biol Interact 171:165–176. doi:10.1016/j.cbi.2006.12.006
Kubinyi H (1999) Chance favors the prepared mind-from serendipity to rational drug design. J Recept Signal Transduct Res 19:15–39. doi:10.3109/10799899909036635
Kiss LE, Ferreira HS, Torrão L, Bonifácio MJ, Palma PN, Soares-da-Silva P et al (2010) Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem 53:3396–3411. doi:10.1021/jm1001524
Learmonth DA, Palma PN, Vieira-Coelho MA, Soares-da-Silva P (2004) Synthesis, biological evaluation, and molecular modeling studies of a novel, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem 47:6207–6217. doi:10.1021/jm040848o
Ferreira JJ, Rocha JF, Santos A, Nunes T, Soares-Da-Silva P (2012) The design of a double-blind, placebo- and active-controlled, multi-national phase-III trial in patients with Parkinson’s disease and end-of-dose motor fluctuations: opicapone superiority vs. placebo and non-inferiority vs. entacapone. Mov Disord 27:365 (abstract)
Dobson CM (2004) Chemical space and biology. Nature 432:824–828. doi:10.1038/nature03192
Ivanenkov YA, Savchuk NP, Ekins S, Balakin KV (2009) Computational mapping tools for drug discovery. Drug Discov Today 14:767–775. doi:10.1016/j.drudis.2009.05.016
Balakin KV, Ivanenkov YA, Savchuk NP (2009) Compound library design for target families. Methods Mol Biol 575:21–46. doi:10.1007/978-1-60761-274-2_2
Reichmann H, Emre M (2012) Optimizing levodopa therapy to treat wearing-off symptoms in Parkinson’s disease: focus on levodopa/carbidopa/entacapone. Expert Rev Neurother 12:119–131. doi:10.1586/ern.11.203
Manson A, Stirpe P, Schrag A (2012) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2:189–198. doi:10.3233/JPD-2012-120103
Fox SH (2013) Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs 73:1405–1415. doi:10.1007/s40265-013-0105-4
The official Web-site of Integrity Database: http://integrity.thomson-pharma.com. Accessed 11 Augt (2014)
Todeschini R, Consonni V (2008) Handbook of molecular descriptors. Wiley-VCH: Verlag GmbH, Weinheim
Kanda T, Jackson MJ, Smith LA, Pearce RK, Nakamura J, Kase H et al (1998) Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol 43:507–513. doi:10.1002/ana.410430415
Perez-Lloret S, Merello M (2014) Two new adenosine receptor antagonists for the treatment of Parkinson’s disease: istradefylline versus tozadenant. Expert Opin Pharmacother 15:1097–1107. doi:10.1517/14656566.2014.903924
Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M (2006) Targeting adenosine \(\text{ A }_{2{\rm A}}\) receptors in Parkinson’s disease. Trends Neurosci 29:647–654. doi:10.1016/j.tins.2006.09.004
Nash JE, Brotchie JM (2000) A common signaling pathway for striatal NMDA and adenosine A2a receptors: implications for the treatment of Parkinson’s disease. J Neurosci 20:7782–7789
Hauser RA, Olanow CW, Kieburtz KD, Neale A, Resburg C, Maya U, et al (2013) A phase 2, placebo-controlled, randomized, double-blind trial of tozadenant (Syn-115) in patients with Parkinson’s disease with wearing-off fluctuations on LD. 21st World Congr Neurol 181 (abstract)
Nyholm D, Johansson A, Lennernäs H, Askmark H (2012) Levodopa infusion combined with entacapone or tolcapone in Parkinson disease: a pilot trial. Eur J Neurol 19:820–826. doi:10.1111/j.1468-1331.2011.03614.x
Marsala SZ, Gioulis M, Ceravolo R, Tinazzi M (2012) A systematic review of catechol-O-methyltransferase inhibitors: efficacy and safety in clinical practice. Clin Neuropharmacol 35:185–190. doi:10.1097/WNF.0b013e31825c034a
Schrag A (2005) Entacapone in the treatment of Parkinson’s disease. Lancet Neurol 4:366–370. doi:10.1016/S1474-4422(05)70098-3
Fernandez HH, Chen JJ (2007) Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy 27:174S–185S. doi:10.1592/phco.27.12part2.174S
Chen JJ, Swope DM (2005) Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease. J Clin Pharmacol 45:878–894. doi:10.1177/0091270005277935
Fariello RG (2007) Safinamide. Neurotherapeutics 4:110–116. doi:10.1016/j.nurt.2006.11.011
Caccia C, Maj R, Calabresi M, Maestroni S, Faravelli L, Curatolo L et al (2006) Safinamide: from molecular targets to a new anti-Parkinson drug. Neurology 67:S18–S23. doi:10.1212/WNL.67.7_suppl_2.S18
Newron Pharmaceuticals reports 2013 financial results. Available at: http://www.zambonpharma.com/it/zspa-newsspa/zc-zcnews/entry/0/1228/2583/newron-pharmaceuticals-reports-2013-financial-results.html. Accessed 11 Augt 2014
Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM (2010) Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther 333:865–873. doi:10.1124/jpet.110.166629
Rascol OO, Lacomblez L, Ferreira J, Negre-Pages L (2007) A ’N=1’ randomized placebo-controlled multiple cross-over pilot study of FP0011, a novel antiglutamate agent, in advanced PD. Mov Disord 22:912 (abstract)
Rees K, Stowe R, Patel S, Ives N, Breen K, Ben-Shlomo Y et al (2011) Anti-hypertensive drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies and clinical trials. Cochrane Database Syst Rev 9:CD008535. doi:10.1002/14651858.CD008535.pub2
Sawada H, Oeda T, Kuno S, Nomoto M, Yamamoto K, Yamamoto M et al (2010) Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS One 5:e15298. doi:10.1371/journal.pone.0015298
Rascol O, Fox S, Gasparini F, Kenney C, Di Paolo T, Gomez-Mancilla B (2014) Use of metabotropic glutamate 5-receptor antagonists for treatment of levodopa-induced dyskinesias. Parkinsonism Relat Disord 20:947–956. doi:10.1016/j.parkreldis.2014.05.003
Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F (2000) The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci Biobehav Rev 24:655–668. doi:10.1016/S0149-7634(00)00028-2
Balaban CD (2002) Neural substrates linking balance control and anxiety. Physiol Behav 77:469–475. doi:10.1016/S0031-9384(02)00935-6
Tohgi H, Abe T, Takahashi S (1993) The effects of L-threo-3,4-dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J Neural Transm Park Dis Dement Sect 5:27–34. doi:10.1007/BF02260912
Barnum CJ, Bhide N, Lindenbach D, Surrena MA, Goldenberg AA, Tignor S et al (2012) Effects of noradrenergic denervation on L-DOPA-induced dyskinesia and its treatment by \(\alpha \)- and \(\beta \)-adrenergic receptor antagonists in hemiparkinsonian rats. Pharmacol Biochem Behav 100:607–615. doi:10.1016/j.pbb.2011.09.009
Lewitt PA, Hauser RA, Lu M, Nicholas AP, Weiner W, Coppard N et al (2012) Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology 79:163–169. doi:10.1212/WNL.0b013e31825f0451
Devilbiss DM, Berridge CW (2006) Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J Pharmacol Exp Ther 319:1327–1335. doi:10.1124/jpet.106.110015
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L et al (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21:RC121
Espay AJ, Dwivedi AK, Payne M, Gaines L, Vaughan JE, Maddux BN et al (2011) Methylphenidate for gait impairment in Parkinson disease: a randomized clinical trial. Neurology 76:1256–1262. doi:10.1212/WNL.0b013e3182143537
Moreau C, Delval A, Defebvre L, Dujardin K, Duhamel A, Petyt G et al (2012) Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol 11:589–596. doi:10.1016/S1474-4422(12)70106-0
Ivachtchenko AV, Ivanenkov YA, Tkachenko SE (2010) 5-Hydroxytryptamine subtype 6 receptor modulators: a patent survey. Expert Opin Ther Pat 20:1171–1196. doi:10.1517/13543776.2010.494661
Loane C, Wu K, Bain P, Brooks DJ, Piccini P, Politis M et al (2013) Serotonergic loss in motor circuitries correlates with severity of action-postural tremor in PD. Neurology 80:1850–1855. doi:10.1212/WNL.0b013e318292a31d
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833. doi:10.1093/brain/awm082
Munoz A, Li Q, Gardoni F, Marcello E, Qin C, Carlsson T et al (2008) Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain 131:3380–3394. doi:10.1093/brain/awn235
Kleedorfer B, Lees AJ, Stern GM, Vanacore N, Meco G (1991) Buspirone in the treatment of LD induced dyskinesias. J Neurol Neurosurg Psychiatry 54:376–377. doi:10.1136/jnnp.54.4.376-a
Amarantus Licenses Phase 2b Ready Eltoprazine For PD-LID. Available at: http://seekingalpha.com/article/1944401-amarantus-licenses-phase-2b-ready-eltoprazine-for-pd-lid. Accessed 11 Augt 2014
van Laar T, De Deyn PP, Aarsland D, Barone P, Galvin JE (2011) Effects of cholinesterase inhibitors in Parkinson’s disease dementia: a review of clinical data. CNS Neurosci Ther 17:428–441. doi:10.1111/j.1755-5949.2010.00166.x
Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ et al (2013) Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 81:1611–1616. doi:10.1212/WNL.0b013e3182a9f558
Chung KA, Lobb BM, Nutt JG, Horak FB (2010) Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 75:1263–1269. doi:10.1212/WNL.0b013e3181f6128c
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M (2008) Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 148:379–397. doi:10.7326/0003-4819-148-5-200803040-00009
Rolinski M, Fox C, Maidment I, McShane R (2012) Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 3:CD006504. doi:10.1002/14651858.CD006504.pub2
Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF (2015) Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 86:135–143. doi:10.1136/jnnp-2014-307659
Chitnis S, Rao J (2009) Rivastigmine in Parkinson’s disease dementia. Expert Opin Drug Metab Toxicol 5:941–955. doi:10.1586/14737175.8.8.1181
Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA (2009) Multiple roles for nicotine in Parkinson’s disease. Biochem Pharmacol 78:677–685. doi:10.1016/j.bcp.2009.05.003
Quik M, Campos C, Grady SR (2013) Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol 86:1153–1162. doi:10.1016/j.bcp.2013.06.027
Russo P, Taly A (2012) \(\alpha \)7-Nicotinic acetylcholine receptors: an old actor for new different roles. Curr Drug Targets 13:574–578. doi:10.2174/138945012800398874
Kawamata J, Suzuki S, Shimohama S (2012) \(\alpha \)7 nicotinic acetylcholine receptor mediated neuroprotection in Parkinson’s disease. Curr Drug Targets 13:623–630. doi:10.2174/138945012800399026
The official Web-site. http://www.talete.mi.it. Accessed 11 Augt 2014
Balakin KV, Etkins S (2009) Pharmaceutical data mining: approaches and applications for drug discovery. Wiley, Hoboken
Trepalin SV, Yarkov AV (2001) CheD:? chemical database compilation tool, internet server, and client for SQL servers. J Chem Inf Comput Sci 41:100–107. doi:10.1002/chin.200120218
Sammon JW (1969) A non-linear mapping for data structure analysis. IEEE Trans Comput C–18:401–409. doi:10.1109/T-C.1969.222678
Pearson K (1901) On lines and planes of closest fit to systems of points in space. Philos Mag 2:559–572. doi:10.1080/14786440109462720
Smith DA (2010) Metabolism, pharmacokinetics and toxicity of functional groups: impact of chemical building blocks on ADMET. RCS, Cambridge
Tan CC, Yu JT, Tan MS et al (2014) Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging 35:941–957. doi:10.1016/j.neurobiolaging.2013.11.019
Ha JY, Kim JS, Kang YH, Bok E, Kim YS, Son JH (2014) Tnfaip8 l1/Oxi-\(\beta \) binds to FBXW5, increasing autophagy through activation of TSC2 in a Parkinson’s disease model. J Neurochem 129:527–538. doi:10.1111/jnc.12643
Verma M, Steer EK, Chu CT (2014) ERKed by LRRK2: a cell biological perspective on hereditary and sporadic Parkinson’s disease. Biochim Biophys Acta 1842:1273–1281. doi:10.1016/j.bbadis.2013.11.00
Estrada AA, Chan BK, Baker-Glenn C et al (2014) Discovery of highly potent, selective, and brain-penetrant aminopyrazole leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J Med Chem 57:921–936. doi:10.1021/jm401654j
Troxler T, Greenidge P, Zimmermann K, Desrayaud S, Drückes P, Schweizer T, Stauffer D, Rovelli G, Shimshek DR (2013) Discovery of novel indolinone-based, potent, selective and brain penetrant inhibitors of LRRK2. Bioorg Med Chem Lett 23:4085–4090. doi:10.1016/j.bmcl.2013.05.054
Conflict of interest
This work was supported by the Ministry of Education and Science of the Russian Federation (02.A03.21.0003; 14.A12.31.0005) and Russian Scientific Fund (14-34-00017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanenkov, Y.A., Veselov, M.S., Chufarova, N.V. et al. Non-dopamine receptor ligands for the treatment of Parkinson’s disease. Insight into the related chemical/property space. Mol Divers 20, 345–365 (2016). https://doi.org/10.1007/s11030-015-9598-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9598-y