Abstract
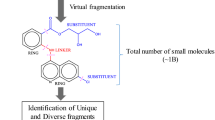
The discovery of novel scaffolds against a specific target has long been one of the most significant but challengeable goals in discovering lead compounds. A scaffold that binds in important regions of the active pocket is more favorable as a starting point because scaffolds generally possess greater optimization possibilities. However, due to the lack of sufficient chemical space diversity of the databases and the ineffectiveness of the screening methods, it still remains a great challenge to discover novel active scaffolds. Since the strengths and weaknesses of both fragment-based drug design and traditional virtual screening (VS), we proposed a fragment VS concept based on Bayesian categorization for the discovery of novel scaffolds. This work investigated the proposal through an application on VEGFR-2 target. Firstly, scaffold and structural diversity of chemical space for 10 compound databases were explicitly evaluated. Simultaneously, a robust Bayesian classification model was constructed for screening not only compound databases but also their corresponding fragment databases. Although analysis of the scaffold diversity demonstrated a very unevenly distribution of scaffolds over molecules, results showed that our Bayesian model behaved better in screening fragments than molecules. Through a literature retrospective research, several generated fragments with relatively high Bayesian scores indeed exhibit VEGFR-2 biological activity, which strongly proved the effectiveness of fragment VS based on Bayesian categorization models. This investigation of Bayesian-based fragment VS can further emphasize the necessity for enrichment of compound databases employed in lead discovery by amplifying the diversity of databases with novel structures.






Similar content being viewed by others
References
Wassermann AM, Kutchukian PS, Lounkine E, Luethi T, Hamon J, Bocker MT, Malik HA, Cowan-Jacob SW, Glick M (2013) Efficient search of chemical space: navigating from fragments to structurally diverse chemotypes. J Med Chem 56:8879–8891. doi:10.1021/jm401309q
Rees DC, Congreve M, Murray CW, Carr R (2004) Fragment-based lead discovery. Nat Rev Drug Discov 3:660–672. doi:10.1038/nrd1467
Congreve M, Chessari G, Tisi D, Woodhead AJ (2008) Recent developments in fragment-based drug discovery. J Med Chem 51:3661–3680. doi:10.1021/jm8000373
Walters WP, Stahl MT, Murcko MA (1998) Virtual screening-an overview. Drug Discov Today 3:160–178. doi:10.1016/S1359-6446(97)01163-X
Kumar A, Voet A, Zhang K (2012) Fragment based drug design: from experimental to computational approaches. Curr Med Chem 19:5128–5147. doi:10.2174/092986712803530467
Pierce AC, Rao G, Bemis GW (2004) BREED: generating novel inhibitors through hybridization of known ligands. Application to CDK2, p38, and HIV protease. J Med Chem 47:2768–2775. doi:10.1021/jm030543u
Bemis GW, Murcko MA (1996) The properties of known drugs. 1. Molecular frameworks. J Med Chem 39:2887–2893. doi:10.1021/jm9602928
Lewell XQ, Judd DB, Watson SP, Hann MM (1998) RECAP retrosynthetic combinatorial analysis procedure: a powerful new technique for identifying privileged molecular fragments with useful applications in combinatorial chemistry. J Chem Inf Comput Sci 38:511–522. doi:10.1021/ci970429i
Schuffenhauer A, Ertl P, Roggo S, Wetzel S, Koch MA, Waldmann H (2007) The scaffold tree-visualization of the scaffold universe by hierarchical scaffold classification. J Chem Inf Model 47:47–58. doi:10.1021/ci600338x
Baker M (2012) Fragment-based lead discovery grows up. Nat Rev Drug Discov 12:5–7. doi:10.1038/nrd3926
Law R, Barker O, Barker JJ, Hesterkamp T, Godemann R, Andersen O, Fryatt T, Courtney S, Hallett D, Whittaker M (2009) The multiple roles of computational chemistry in fragment-based drug design. J Comput Aided Mol Des 23:459–473. doi:10.1007/s10822-009-9284-1
Yuan H, Tai W, Hu S, Liu H, Zhang Y, Yao S, Ran T, Lu S, Ke Z, Xiong X (2013) Fragment-based strategy for structural optimization in combination with 3D-QSAR. J Comput Aided Mol Des 27:897–915. doi:10.1007/s10822-013-9687-x
Hou T, Xu X (2004) Recent development and application of virtual screening in drug discovery: an overview. Curr Pharm Des 10:1011–1033. doi:10.2174/1381612043452721
Xia X, Maliski EG, Gallant P, Rogers D (2004) Classification of kinase inhibitors using a Bayesian model. J Med Chem 47:4463–4470. doi:10.1021/jm0303195
Xie Q-Q, Zhong L, Pan Y-L, Wang X-Y, Zhou J-P, Di-wu L, Huang Q, Wang Y-L, Yang L-L, Xie H-Z (2011) Combined SVM-based and docking-based virtual screening for retrieving novel inhibitors of c-Met. Eur J Med Chem 46:3675–3680. doi:10.1016/j.ejmech.2011.05.031
Singh N, Chaudhury S, Liu R, AbdulHameed MDM, Tawa G, Wallqvist A (2012) QSAR classification model for antibacterial compounds and its use in virtual screening. J Chem Inf Model 52:2559–2569. doi:10.1021/ci300336v
Vijayan R, Bera I, Prabu M, Saha S, Ghoshal N (2009) Combinatorial library enumeration and lead hopping using comparative interaction fingerprint analysis and classical 2D QSAR methods for seeking novel GABAA \({\alpha } 3\) modulators. J Chem Inf Model 49:2498–2511. doi:10.1021/ci900309s
Lee JH, Lee S, Choi S (2010) In silico classification of adenosine receptor antagonists using Laplacian-modified naive Bayesian, support vector machine, and recursive partitioning. J Mol Graph Model 28:883–890. doi:10.1016/j.jmgm.2010.03.008
Prathipati P, Ma NL, Keller TH (2008) Global Bayesian models for the prioritization of antitubercular agents. J Chem Inf Model 48:2362–2370. doi:10.1021/ci800143n
Wang S, Li Y, Wang J, Chen L, Zhang L, Yu H, Hou T (2012) ADMET evaluation in drug discovery. 12. Development of binary classification models for prediction of hERG potassium channel blockage. Mol Pharm 9:996–1010. doi:10.1021/mp300023x
Hu Y, Unwalla R, Denny RA, Bikker J, Di L, Humblet C (2010) Development of QSAR models for microsomal stability: identification of good and bad structural features for rat, human and mouse microsomal stability. J Comput Aided Mol Des 24:23–35. doi:10.1007/s10822-009-9309-9
Kombo DC, Bencherif M (2013) Comparative study on the use of Docking and Bayesian categorization to predict ligand binding to nicotinic acetylcholine receptors (nAChRs) subtypes. J Chem Inf Model 53:3212–3222. doi:10.1021/ci400493a
Yuan H, Lu T, Ran T, Liu H, Lu S, Tai W, Leng Y, Zhang W, Wang J, Chen Y (2011) Novel strategy for three-dimensional fragment-based lead discovery. J Chem Inf Model 51:959–974. doi:10.1021/ci200003c
Kiselyov A, Balakin KV, Tkachenko SE (2007) VEGF/VEGFR signalling as a target for inhibiting angiogenesis. Expert Opin Investig Drugs 16:83–107. doi:10.1517/13543784.16.1.83
Huang L, Huang Z, Bai Z, Xie R, Sun L, Lin K (2012) Development and strategies of VEGFR-2/KDR inhibitors. Future Med Chem 4:1839–1852. doi:10.4155/fmc.12.121
Boyer SJ (2002) Small molecule inhibitors of KDR (VEGFR-2) kinase: an overview of structure activity relationships. Curr Top Med Chem 2:973–1000. doi:10.2174/1568026023393273
Musumeci F, Radi M, Brullo C, Schenone S (2012) Vascular endothelial growth factor (VEGF) receptors: drugs and new inhibitors. J Med Chem 55:10797–10822. doi:10.1021/jm301085w
Zhang Y, Liu H, Jiao Y, Yuan H, Wang F, Lu S, Yao S, Ke Z, Tai W, Jiang Y (2012) De novo design of N-(pyridin-4-ylmethyl) aniline derivatives as KDR inhibitors: 3D-QSAR, molecular fragment replacement, protein-ligand interaction fingerprint, and ADMET prediction. Mol Divers 16:787–802. doi:10.1007/s11030-012-9405-y
Zhang Y, Yang S, Jiao Y, Liu H, Yuan H, Lu S, Ran T, Yao S, Ke Z, Xu J (2013) An Integrated Virtual Screening Approach for VEGFR-2 Inhibitors. J Chem Inf Model 53:3163–3177. doi:10.1021/ci400429g
Socinski MA (2011) Multitargeted receptor tyrosine kinase inhibition: an antiangiogenic strategy in non-small cell lung cancer. Cancer Treat Rev 37:611–617. doi:10.1016/j.ctrv.2011.04.003
Langdon SR, Brown N, Blagg J (2011) Scaffold diversity of exemplified medicinal chemistry space. J Chem Inf Model 51:2174–2185. doi:10.1021/ci2001428
Shen M, Tian S, Li Y, Li Q, Xu X, Wang J, Hou T (2012) Drug-likeness analysis of traditional Chinese medicines: 1. property distributions of drug-like compounds, non-drug-like compounds and natural compounds from traditional Chinese medicines. J Cheminformatics 4:1–13. doi:10.1186/1758-2946-4-31
Mysinger MM, Carchia M, Irwin JJ, Shoichet BK (2012) Directory of useful decoys, enhanced (DUD-E): better ligands and decoys for better benchmarking. J Med Chem 55:6582–6594. doi:10.1021/jm300687e
Inc. AS (2008) Pilot Pipeline version 7.5. Accelrys Software Inc: San Diego
Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008) DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res 36:D901–D906. doi:10.1093/nar/gkm958
Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, Maciejewski A, Arndt D, Wilson M, Neveu V (2014) DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res 42:D1091–D1097. doi:10.1093/nar/gkt1068
McGregor MJ, Pallai PV (1997) Clustering of large databases of compounds: using the MDL “keys” as structural descriptors. J Chem Inf Comput Sci 37:443–448. doi:10.1021/ci960151e
Butina D (1999) Unsupervised data base clustering based on daylight’s fingerprint and Tanimoto similarity: a fast and automated way to cluster small and large data sets. J Chem Inf Comput Sci 39:747–750. doi:10.1021/ci9803381
Leeson P (2012) Drug discovery: chemical beauty contest. Nature 481:455–456. doi:10.1038/481455a
Ihlenfeldt W-D, Voigt JH, Bienfait B, Oellien F, Nicklaus MC (2002) Enhanced CACTVS browser of the open NCI database. J Chem Inf Comput Sci 42:46–57. doi:10.1021/ci010056s
Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG (2012) ZINC: a free tool to discover chemistry for biology. J Chem Inf Model 52:1757–1768. doi:10.1021/ci3001277
Zauhar RJ, Gianti E, Welsh WJ (2013) Fragment-based shape signatures: a new tool for virtual screening and drug discovery. J Comput Aided Mol Des 27:1009–1036. doi:10.1007/s10822-013-9698-7
Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B (2012) ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res 40:D1100–D1107. doi:10.1093/nar/gkr777
van Linden OP, Kooistra AJ, Leurs R, de Esch IJ, de Graaf C (2013) KLIFS: a knowledge-based structural database to navigate kinase-ligand interaction space. J Med Chem 57:249–277. doi:10.1021/jm400378w
Kirchmair J, Markt P, Distinto S, Schuster D, Spitzer GM, Liedl KR, Langer T, Wolber G (2008) The Protein Data Bank (PDB), its related services and software tools as key components for in silico guided drug discovery. J Med Chem 51:7021–7040. doi:10.1021/jm8005977
Mauser H, Stahl M (2007) Chemical fragment spaces for de novo design. J Chem Inf Model 47:318–324. doi:10.1021/ci6003652
Shneiderman B (1992) Tree visualization with tree-maps: 2-d space-filling approach. ACM Trans Graph 11:92–99. doi:10.1145/102377.115768
Krier M, Bret G, Rognan D (2006) Assessing the scaffold diversity of screening libraries. J Chem Inf Model 46:512–524. doi:10.1021/ci050352v
Kibbey C, Calvet A (2005) Molecular Property eXplorer: a novel approach to visualizing SAR using tree-maps and heatmaps. J Chem Inf Model 45:523–532. doi:10.1021/ci0496954
Clark AM (2009) 2D depiction of fragment hierarchies. J Chem Inf Model 50:37–46. doi:10.1021/ci900350h
Cross JB, Thompson DC, Rai BK, Baber JC, Fan KY, Hu Y, Humblet C (2009) Comparison of several molecular docking programs: pose prediction and virtual screening accuracy. J Chem Inf Model 49:1455–1474. doi:10.1021/ci900056c
Kontoyianni M, McClellan LM, Sokol GS (2004) Evaluation of docking performance: comparative data on docking algorithms. J Med Chem 47:558–565. doi:10.1021/jm0302997
Fraley ME, Arrington KL, Buser CA, Ciecko PA, Coll KE, Fernandes C, Hartman GD, Hoffman WF, Lynch JJ, McFall RC (2004) Optimization of the indolyl quinolinone class of KDR (VEGFR-2) kinase inhibitors: effects of 5-amido-and 5-sulphonamido-indolyl groups on pharmacokinetics and hERG binding. Bioorg Med Chem Lett 14:351–355. doi:10.1016/j.bmcl.2003.11.007
Dinges J, Akritopoulou-Zanze I, Arnold LD, Barlozzari T, Bousquet PF, Cunha GA, Ericsson AM, Iwasaki N, Michaelides MR, Ogawa N (2006) Hit-to-lead optimization of 1, 4-dihydroindeno [1, 2-c] pyrazoles as a novel class of KDR kinase inhibitors. Bioorg Med Chem Lett 16:4371–4375. doi:10.1016/j.bmcl.2006.05.052
Akritopoulou-Zanze I, Albert DH, Bousquet PF, Cunha GA, Harris CM, Moskey M, Dinges J, Stewart KD, Sowin TJ (2007) Synthesis and biological evaluation of 5-substituted 1, 4-dihydroindeno [1, 2-c] pyrazoles as multitargeted receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett 17:3136–3140. doi:10.1016/j.bmcl.2007.03.031
Dinges J, Ashworth KL, Akritopolou-Zanze I, Arnold LD, Baumeister SA, Bousquet PF, Cunha GA, Davidsen SK, Djuric SW, Gracias VJ (2006) 1, 4-Dihydroindeno [1, 2-c] pyrazoles as novel multitargeted receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett 16:4266–4271. doi:10.1016/j.bmcl.2006.05.066
Dinges J, Albert DH, Arnold LD, Ashworth KL, Akritopoulou-Zanze I, Bousquet PF, Bouska JJ, Cunha GA, Davidsen SK, Diaz GJ (2007) 1, 4-Dihydroindeno [1, 2-c] pyrazoles with acetylenic side chains as novel and potent multitargeted receptor tyrosine kinase inhibitors with low affinity for the hERG ion channel. J Med Chem 50:2011–2029. doi:10.1021/jm061223o
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (81302634, 21302225 and NSFC81473077), the Fundamental Research Funds for the Central Universities (PT2014LX0072); the Natural Science Foundation of Jiangsu Province (BK20130662), the Postgraduate Innovative Foundation supported by Jiangsu Province (KYLX_0637), and the Jiangsu Qinglan Project.
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Jiao, Y., Xiong, X. et al. Fragment virtual screening based on Bayesian categorization for discovering novel VEGFR-2 scaffolds. Mol Divers 19, 895–913 (2015). https://doi.org/10.1007/s11030-015-9592-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9592-4