Abstract
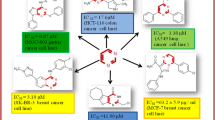
Withaferin-A (WA) has attracted the attention of chemists as well as biologists due to its interesting structure and various bio-activities. In light of the promising biological importance of WA as well as pyrrolidine-2-spiro-3\(^{\prime }\)-oxindole ring system, we became interested in the synthesis of a combined motif involving both the ring systems via the 1,3-dipolar cycloaddition of WA at \(\Delta ^{2}\)-bond of the \(\upalpha \),\(\upbeta \)-unsaturated carbonyl system. We now report a facile, atom-economic synthesis of novel spiro-pyrrolizidino-oxindole adducts of withaferin-A (10 compounds) via the intermolecular cycloaddition of azomethine ylides generated in situ from proline and isatins/acenaphthoquinone. The reaction is highly chemo, regio, and stereoselective affording the cis-fused products with \(\upbeta \)-orienting hydrogen. The structures were determined by 1D/2D NMR spectroscopic data analysis and unequivocally confirmed by X-ray crystallographic analysis in some cases. Bioevaluation of the compounds against six cancer lines (e.g., CHO, HepG2, HeLa, HEK 293, MDCK-II, and Caco-2) identified 4 promising potential anticancer compounds.
Graphical Abstract








Similar content being viewed by others
References
Lavie D, Glotter E, Shvo Y (1965) Constituents of Withania somnifera Dun: III. The side chain of withaferin A. J Org Chem 30:1774–1778. doi:10.1021/jo01017a015
Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS (2004) Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 7:115–122
Yokota Y, Bargagna-Mohan P, Ravindranath PP, Kim KB, Mohan R (2006) Development of withaferin A analogs as probes of angiogenesis. Bioorg Med Chem Lett 16:2603–2607. doi:10.1016/j.bmcl.2006.02.039
Kumar PS, Shilpa P, Salimath BP (2009) Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor. Curr Trends Biotechnol Pharm 3:138–148
Jilani K, Lupescu A, Zbidah M, Shaik N, Lang F (2013) Withaferin A-stimulated \(\text{ Ca }^{2+}\) entry, ceramide formation and suicidal death of erythrocytes. Toxicol Vitro 27:52–58. doi:10.1016/j.tiv.2012.09.004
Wanjari P, Jayadeepa RM (2012) A novel in-silico drug designing approach for identification of natural compounds for treatment of hypothyroid. Int Proc Chem Biol Environ Eng 31:12–16
Khedgikar V, Kushwaha P, Gautam J, Verma A, Changkija B, Kumar A, Sharma S, Nagar GK, Singh D, Trivedi PK, Sangwan NS, Mishra PR, Trivedi R (2013) Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis 4:e778. doi:10.1038/cddis.2013.294
Antony ML, Lee J, Hahm ER, Kim SH, Marcus AL, Kumari V, Ji X, Yang Z, Vowell CL, Wipf P, Uechi GT, Yates NA, Romero G, Sarkar SN, Singh SV (2014) Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of \(\upbeta \)-tubulin. J Bio Chem 289:1852–1865. doi:10.1074/jbc.M113.496844
Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D (2007) Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res 67:246–253. doi:10.1158/0008-5472.CAN-06-2430
Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH, Lee JM, Kim YH, Park JW, Kwon TK (2008) Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 13:1494–1504. doi:10.1007/s10495-008-0273-y
Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C (2011) Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis 16:1014–1027. doi:10.1007/s10495-011-0625-x
Munagala R, Kausar H, Munjal C, Gupta CR (2011) Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 32:1697–1705. doi:10.1093/carcin/bgr192
Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C (2010) Notch-1 inhibition by withaferin-A: a therapeutic target against colon carcinogenesis. Mol Canc Therapeut 9:202–210. doi:10.1158/1535-7163.MCT-09-0771
Kaileh M, Berghe VW, Heyerick A, Horion J, Piette J, Libert C, Keukeleire DD, Essawi T, Haegeman G (2007) Withaferin A strongly elicits I\(\upkappa \)B kinase \(\upbeta \) hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Bio Chem 282:4253–4264. doi:10.1074/jbc.M606728200
Khan ZA, Ghosh AR (2010) Possible nitric oxide modulation in protective effects of Withaferin A against stress induced neurobehavioural changes. J Med Plants Res 4:490–495. doi:10.5897/JMPR09.079
Khan ZA, Ghosh AR (2010) Involvement of nNOS in the antidepressant-like effect of Withaferin-A in rats. J Pharm Biomed Sci 7:1–2
Gupta SK, Mohanty I, Talwar KK, Dinda A, Joshi S, Bansal P, Saxena A, Arya DS (2004) Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem 260:39–47. doi:10.1023/B:MCBI.0000026051.16803.03
Agarwal R, Diwanay S, Patki P, Patwardhan B (1999) Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J Ethnopharmacol 67:27–35. doi:10.1016/S0378-8741(99)00065-3
Yang H, Wang Y, Cheryan VT, Wu W, Cui CQ, Polin LA, Pass HI, Dou QP, Rishi AK, Wali A (2012) Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo. PLoS One 7:e41214. doi:10.1371/journal.pone.0041214
Min KJ, Choi K, Kwon TK (2011) Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. Int Immunopharmacol 11:1137–1142. doi:10.1016/j.intimp.2011.02.029
Devi PU, Kamath R (2003) Radiosensitizing effect of withaferin A combined with hyperthermia on mouse fibrosarcoma and melanoma. J Radiat Res 44:1–6
Amslinger S (2010) The tunable functionality of \(\upalpha \),\(\upbeta \)-unsaturated carbonyl compounds enables their differential application in biological systems. ChemMedChem 5:351–356. doi:10.1002/cmdc.200900499
Fuska J, Prousek J, Rosazza J, Budesinsky M (1982) Microbial transformations of natural antitumor agents, 23. Conversion of withaferin-A to 12\(\upbeta \)- and 15\(\upbeta \)- hydroxy derivatives of withaferin-A. Steroids 40:157–169. doi:10.1016/0039-128X(82)90030-7
Rahman AU, Farooq A, Anjum S, Choudhary MI (1999) Microbial transformation of cytotoxic natural products. Curr Org Chem 3:309–326
Rosazza JP, Nicholas AW, Gustafson ME (1978) Microbial transformations of natural antitumor agents. 7. 14-alpha-hydroxylation of withaferin-a by cunninghamella elegans (NRRL 1393). Steroids 31:671–679. doi:10.1016/S0039-128X(78)80007-5
Motiwala HF, Bazzill J, Samadi A, Zhang H, Timmermann BN, Cohen MS, Aube J (2013) Synthesis and cytotoxicity of semisynthetic withalongolide a analogues. ACS Med Chem Lett 4:1069–1073. doi:10.1021/ml400267q
Joshi P, Misra L, Siddique AA, Srivastava M, Kumar S, Darkor MP (2014) Epoxide group relationship with cytotoxicity in withanolide derivatives from Withania somnifera. Steroids 79:19–27. doi:10.1016/j.steroids.2013.10.008
Misra L, Lal P, Chaurasia ND, Sangwan RS, Sinha S, Tuli R (2008) Selective reactivity of 2-mercaptoethanol with 5\(\upbeta \),6\(\upbeta \)-epoxide in steroids from Withania somnifera. Steroids 73:245–251. doi:10.1016/j.steroids.2007.10.006
Yousuf SK, Majeed R, Ahmad M, Sangwan P, Purnima B, Saxena AK, Suri KA, Mukherjee D, Taneja SC (2011) Ring A structural modified derivatives of withaferin A and the evaluation of their cytotoxic potential. Steroids 76:1213–1222. doi:10.1016/j.steroids.2011.05.012
Nicholas AW, Rosazza JP (1976) Reactions of withaferin-A with model biological nucleophiles. Biorg Chem 5:367–372. doi:10.1016/0045-2068(76)90021-3
Jossang A, Jossang P, Hadi HA, Sevenet T, Bodo B (1991) Horsfiline, an oxindole alkaloid from Horsfieldia superb. J Org Chem 56:6527–6530. doi:10.1021/jo00023a016
Sebahar PR, Williams RM (2000) The asymmetric total synthesis of (+)- and (\(-\))-spirotryprostatin B. J Am Chem Soc 122:5666–5667. doi:10.1021/ja001133n
Meyers C, Carreira EM (2003) Total synthesis of (\(-\))-spirotryprostatin B. Angew Chem Int Ed 42:694–696. doi:10.1002/anie.200390192
Hilton ST, Ho TCT, Pljevaljcic G, Jones K (2000) A new route to spirooxindoles. Org Lett 2:2639–2641. doi:10.1021/ol0061642
Amornraksa K, Grigg R, Gunaratna HQN, Kemp J, Sridharan V (1987) X = Y\(-\)ZH Systems as potential 1,3-dipoles: part 8. Pyrrolidines and \(\Delta ^{5}\)-pyrrolines (3,7-diazabicyclo[3.3.0]octenes) from the reaction of imines of \(\upalpha \)-amino acids and their esters with cyclic dipolarophiles. Mechanism of racemisation of \(\upalpha \)-amino acids and their esters in the presence of aldehydes. J Chem Soc Perkin Trans 1:2285–2296. doi:10.1039/P19870002285
Grigg R, Thianpatanaqul S (1984) Decarboxylative transamination. Mechanism and applications to the synthesis of heterocyclic compounds. J Chem Soc Chem Commun 180–181. doi:10.1039/C39840000180
Misra L, Lal P, Sangwan RS, Sangwan NS, Uniyal GC, Tuli R (2005) Unusually sulphated and oxygenated steroids from Withania somnifera. Phytochemistry 66:2702–2707. doi:10.1016/j.phytochem.2005.10.001
Talupula BK (2011) Cytotoxicity of PBN spin trap on A204 cells. J Adv Pharm Res 2:9–17
Al-Qubaisi M, Rozita R, Yeap SK, Omar AR, Ali AM, Alitheen NB (2011) Selective cytotoxicity of goniothalamin against hepatoblastoma HepG2 Cells. Molecules 16:2944–2959. doi:10.3390/molecules16042944
Acknowledgments
The authors express their gratitude to the Director, IICB for laboratory facilities. This work received financial assistance from the Council of Scientific and Industrial Research Government of India. B.Y., S.K., N. S., A.H., and N.B.M. are recipients of Research Fellowships and Emeritus Scientist grant from CSIR. Our thanks are due to Dr. B. Achari (Ex-emeritus Scientist, CSIR) for helpful suggestions and Dr. R. Natarajan (Senior Scientist) and Dr. Prakas R. Maulik (Emeritus Scientist, CSIR) and Mr. Sandip Kundu of IICB for helping in X-ray structure solution and Mr. E. Padmanaban for recording NMR spectrometric data.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bharitkar, Y.P., Kanhar, S., Suneel, N. et al. Chemistry of withaferin-A: chemo, regio, and stereoselective synthesis of novel spiro-pyrrolizidino-oxindole adducts of withaferin-A via one-pot three-component [3+2] azomethine ylide cycloaddition and their cytotoxicity evaluation. Mol Divers 19, 251–261 (2015). https://doi.org/10.1007/s11030-015-9574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9574-6