Abstract
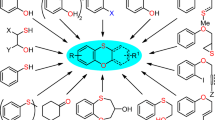
A variety of organocatalysts has been screened for the synthesis of arylaminonaphthols. It has been shown that (N,N-dimethylethanolamine) is a highly efficient organocatalyst for the direct synthesis of a novel class of arylaminonaphthols via three-component condensation of 2-naphthol, aldehydes, and arylamines under solvent-free conditions. Mild, one-pot, and green reaction conditions, relatively short reaction times and good yields make this protocol highly significant. 25 new compounds have been synthesized by this method.







Similar content being viewed by others
References
Knapp S (1995) Synthesis of complex nucleoside antibiotics. Chem Rev 95:1859–1876. doi:10.1021/cr00038a006
Mannhold R, Kubinyi H (2006) Nucleic acid drugs. In: Dingermann T, Steinhilber D, Folkers G (eds) Molecular biology in medicinal chemistry. Wiley, Weinheim. doi:10.1002/3527602666
Wang YF, Izawa T, Kobayashi S, Ohno M (1982) Stereocontrolled synthesis of (+)-negamycin from an acyclic homoallylamine by 1,3-asymmetric induction. J Am Chem Soc 104:6465–6466. doi:10.1021/ja00387a060
Cardellicchio C, Capozzi MAM, Naso F (2010) The Betti base: the awakening of a sleeping beauty. Tetrahedron 21:507–517. doi:10.1016/j.tetasy.2010.03.020
Elmendorf HG, Walls CD, Wolf C (2012) Compositions and methods for the treatment of giardiasis. US Patent 20,120,283,267
Gerlach M, Maul C (2007) Substituted 1 and 2-naphthol Mannich bases. US Patent 7,202,242 B2
Gyemant N, Engi H, Schelz Z, Szatmari I, Toth D, Fulop F, Molnar J, de Witte P (2010) In vitro and in vivo multidrug resistance reversal activity by a Betti-base derivative of tylosin. Br J Cancer 103:178–185. doi:10.1038/sj.bjc.6605716
Shen AY, Tsai CT, Chen CL (1999) Synthesis and cardiovascular evaluation of \(N\)-substituted 1-aminomethyl-2-naphthols. Eur J Med Chem 34:877–882. doi: 10.1016/S0223-5234(99)00204-4
Szatmári I, Fülöp F (2013) Syntheses, transformations and applications of aminonaphthol derivatives prepared via modified Mannich reactions. Tetrahedron 69:1255–1278. doi:10.1016/j.tet.2012.11.055
Salama TA (2013) Silicon-induced general, mild, and efficient one-pot, three-component synthesis of amidoalkyl naphthol libraries. Synlett 24:713–718. doi:10.1055/s-0032-1318392
Safari J, Zarnegar Z (2013) A magnetic nanoparticle-supported sulfuric acid as a highly efficient and reusable catalyst for rapid synthesis of amidoalkyl naphthols. J Mol Catal A 379:269–276. doi:10.1016/j.molcata.2013.08.028
Rostamizadeh S, Abdollahi F, Shadjou N, Amani AM (2013) MCM-41-SO\(_{3}\)H: a novel reusable nanocatalyst for synthesis of amidoalkyl naphthols under solvent-free conditions. Monatsh Chem 144:1191–1196. doi: 10.1007/s00706-013-0936-4
Lei ZK, Xiao L, Lu XQ, Huang H, Liu CJ (2013) Graphite supported perchloric acid (HClO\(_{4}\)-C): an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Molecules 18:1653–1659. doi: 10.3390/molecules18021653
Das VK, Borah M, Thakur AJ (2013) Piper-betle-shaped nano-S-catalyzed synthesis of 1-amidoalkyl-2-naphthols under solvent-free reaction condition: A greener “Nanoparticle-Catalyzed Organic Synthesis Enhancement” approach. J Org Chem 78:3361–3366. doi:10.1021/jo302682k
Csutortoki R, Szatmari I, Fulop F (2013) Syntheses of amido-, carbamido- and carbamatoalkylnaphthols. Curr Org Synth 10:564–583
Mulla SA, Salama TA, Pathan MY, Inamdar SM, Chavan SS (2012) Solvent-free, highly efficient one-pot multi-component synthesis of 1-amido and 1-carbamato-alkyl naphthols/phenols catalyzed by ethylammonium nitrate as reusable ionic liquid under neat reaction condition at ambient temperature. Tetrahedron Lett 54:672–675. doi:10.1016/j.tetlet.2012.12.004
Kotadia DA, Soni SS (2012) Silica gel supported-SO\(_{3}\)H functionalised benzimidazolium based ionic liquid as a mild and effective catalyst for rapid synthesis of 1-amidoalkyl naphthols. J Mol Catal A 353:44–49. doi: 10.1016/j.molcata.2011.11.003
Zolfigol MA, Khazaei A, Moosavi-Zare AR, Zare A, Khakyzadeh V (2011) Rapid synthesis of 1-amidoalkyl-2-naphthols over sulfonic acid functionalized imidazolium salts. Appl Catal A 400:70–81. doi:10.1016/j.apcata.2011.04.013
Tamaddon F, Bistgani JM (2011) \([\text{ MeC(OH) }_{2}]^{+}\text{ ClO }_{4}^{-}\): a new efficient organocatalyst for the preparation of 1-amido-and 1-carbamato-alkyl naphthols. Synlett 2011:2947–2950. doi:10.1055/s-0031-1289906
Mistry SR, Joshi RS, Maheria KC (2011) Zeolite H-BEA catalysed multicomponent reaction: one-pot synthesis of amidoalkyl naphthols—biologically active drug-like molecules. J Chem Sci 123:427–432. doi:10.1007/s12039-011-0095-2
Shaterian HR, Yarahmadi H, Ghashang M (2008) Silica supported perchloric acid (HClO\(_{4}\)-SiO\(_{2})\): an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Tetrahedron 64:1263–1269. doi: 10.1016/j.tet.2007.11.070
Shaterian HR, Yarahmadi H, Ghashang M (2008) An efficient, simple and expedition synthesis of 1-amidoalkyl-2-naphthols as “drug like” molecules for biological screening. Bioorg Med Chem Lett 18:788–792. doi:10.1016/j.tet.2007.11.070
Karmakar B, Banerji J (2011) A competent pot and atom-efficient synthesis of Betti bases over nanocrystalline MgO involving a modified Mannich type reaction. Tetrahedron Lett 52:4957–4960. doi:10.1016/j.tetlet.2011.07.075
Periasamy M, Anwar S, Reddy MN (2009) Simple and convenient methods for synthesis, resolution and application of aminonaphthols. Indian J Chem B 48:1261–1273
Ghandi M, Olyaei A, Raoufmoghaddam S (2008) One-pot, three-component uncatalyzed quantitative synthesis of new aminonaphthols (Betti bases) in water. Synth Commun 38:4125–4138. doi:10.1080/00397910802279860
Olyaei A, Raoufmoghaddam S, Sadeghpour M, Ebadzadeh B (2010) Convenient and efficient method for the synthesis of \(N\)-heteroaryl aminonaphthols under solvent-free conditions. Chin J Chem 28:825–832. doi: 10.1002/cjoc.201090153
Hosseinian A, Shaterian HR (2012) \(\text{ NaHSO }_{4} \cdot \text{ H }_{2}\text{ O }\) catalyzed multicomponent synthesis of 1-(benzothiazolylamino) methyl2-naphthols under solvent-free conditions. Phosphorus Sulfur Silicon 187:1056–1063. doi: 10.1080/10426507.2012.664221
Buckley BR, Neary SP (2010) Organocatalysis. Annu Rep Prog Chem Sect B Org Chem 106:120–135. doi:10.1039/B927086H
Grondal C, Jeanty M, Enders D (2010) Organocatalytic cascade reactions as a new tool in total synthesis. Nat Chem 2:167–178. doi:10.1038/nchem.539
Marcelli T, van Maarseveen JH, Hiemstra H (2006) Cupreines and cupreidines: an emerging class of bifunctional cinchona organocatalysts. Angew Chem Int Ed 45:7496–7504. doi:10.1002/anie.200602318
Liu G, Zhang S, Li H, Zhang T, Wang W (2011) Organocatalytic enantioselective Friedel–Crafts reactions of 1-naphthols with aldimines. Org Lett 13:828–831. doi:10.1021/ol102987n
Ramachary DB, Babul Reddy G (2007) A new organocatalyst for Friedel–Crafts alkylation of 2-naphthols with isatins: application of an organo-click strategy for the cascade synthesis of highly functionalized molecules. Tetrahedron Lett 48:7618–7623. doi:10.1016/j.tetlet.2007.08.129
Shahrisa A, Safa KD, Esmati S (2014) Synthesis, spectroscopic and DFT studies of novel fluorescent dyes: 3-aminoimidazo[1,2-a]pyridines possessing 4-pyrone moieties. Spectrochim Acta A 117:614–621. doi:10.1016/j.saa.2013.09.056
Shahrisa A, Esmati S, Miri R, Firuzi O, Edraki N, Nejati M (2013) Cytotoxic activity assessment, QSAR and docking study of novel bis-carboxamide derivatives of 4-pyrones synthesized by Ugi four-component reaction. Eur J Med Chem 66:388–399. doi:10.1016/j.ejmech.2013.05.049
Shahrisa A, Esmati S (2013) Three novel sequential reactions for the facile synthesis of a library of bisheterocycles possessing the 3-aminoimidazo [1,2-a] pyridine core catalyzed by bismuth (III) chloride. Synlett 24:595–602. doi:10.1055/s-0032-1318221
Shahrisa A, Miri R, Esmati S, Saraei M, Mehdipour AR, Sharifi M (2012) Synthesis and calcium channel antagonist activity of novel 1,4-dihydropyridine derivatives possessing 4-pyrone moieties. Med Chem Res 21:284–292. doi:10.1007/s00044-010-9534-8
Shahrisa A, Esmati S, Nazari MG (2012) Boric acid as a mild and efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. J Chem Sci 124:927–931. doi:10.1007/s12039-012-0285-6
Dindulkar SD, Puranik VG, Jeong YT (2012) Supported copper triflate as an efficient catalytic system for the synthesis of highly functionalized 2-naphthol Mannich bases under solvent free condition. Tetrahedron Lett 53:4376–4380. doi:10.1016/j.tetlet.2012.06.022
Ganesan SS, Rajendran N, Sundarakumar SI, Ganesan A, Pemiah B (2013) \(\beta \)-Naphthol in glycerol: a versatile pair for efficient and convenient synthesis of aminonaphthols, naphtho-1,3-oxazines, and benzoxanthenes. Synthesis 45:1564–1568. doi: 10.1055/s-0033-1338430
Saidi MR, Azizi N, Naimi-Jamal MR (2001) Lithium perchlorate assisted one-pot three-component aminoalkylation of electron-rich aromatic compounds. Tetrahedron Lett 42:8111–8113. doi:10.1016/S0040-4039(01)01732-4
Katritzky AR, Abdel-Fattah AA, Tymoshenko DO, Belyakov SA, Ghiviriga I, Steel PJ (1999) Amino (hetero) arylmethylation of phenols with \(N\)-[\(\alpha \)-amino (hetero) arylmethyl] benzotriazoles. J Org Chem 64:6071–6075. doi:10.1021/jo9903609
Kumar A, Gupta MK, Kumar M (2010) Non-ionic surfactant catalyzed synthesis of Betti base in water. Tetrahedron Lett 51:1582–1584. doi:10.1016/j.tetlet.2010.01.056
Acknowledgments
We thank research affairs of the University of Tabriz for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shahrisa, A., Teimuri-Mofrad, R. & Gholamhosseini-Nazari, M. Synthesis of a new class of Betti bases by the Mannich-type reaction: efficient, facile, solvent-free and one-pot protocol. Mol Divers 19, 87–101 (2015). https://doi.org/10.1007/s11030-014-9559-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9559-x