Abstract
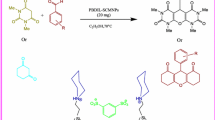
Mo(IV) salen complex (2.5 mol%) was found to be a highly efficient catalyst for the one-pot synthesis of 2,4,5-triarylimidazoles via a three-component reaction using benzil or benzoin, aryl aldehydes, and ammonium acetate as a nitrogen source under mild conditions. In order to recover and the reuse of the catalyst, a new Mo(IV) salen–silica nanoparticle as heterogeneous catalyst was prepared by simple and successful immobilization of the catalyst onto silica (3-aminopropyl functionalized silica gel). This procedure can be applied to large-scale conditions with high efficiency. Experimental evidence showed that the catalyst is stable and can be easily recovered and reused for at least five times without significant loss of activity. The nanocatalyst was characterized using FT-IR spectroscopy, scanning electron microscopy, atomic force microscopy, powder X-ray diffraction , transmission electron microscopy, thermogravimetric instrument for analysis of nitrogen adsorption, and inductively coupled plasma spectrometer.
Graphical Abstract





Similar content being viewed by others
References
Samai S, Nandi GC, Singh P, Singh MS (2009) L-Proline: an efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron 65:10155–10161. doi:10.1016/j.tet.2009.10.019
Lambardino JG, Wiseman EH (1974) Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. J Med Chem 17:1182–1188. doi:10.1021/jm00257a011
Gadekar LS, Mane SR, Katkar SS, Arbad BR, Lande MK (2009) Scolecite as an efficient heterogeneous catalyst for the synthesis of 2,4,5-triarylimidazoles. Cent Eur J Chem 7:550–554. doi:10.2478/s11532-009-0050-y
Balalaie S, Arabanian A (2000) One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem 2:274–276. doi:10.1039/b006201o
Wang L, Cai C (2009) Polymer-supported zinc chloride: a highly active and reusable heterogeneous catalyst for one-pot synthesis of 2,4,5-trisubstituted imidazoles. Monatsh Chem 140:541–546. doi:10.1007/s00706-008-0086-2
Sparks RB, Combs AP (2004) Microwave-assisted synthesis of 2,4,5-triaryl-imidazole; a novel thermally induced N-hydroxyimidazole N–O bond cleavage. Org Lett 6:2473–2475. doi:10.1021/ol049124x
Sharma D, Hazarika P, Konwar D (2008) An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by \(\text{ InCl }_{3}.\text{3H }_{2}\text{ O }\). Tetrahedron Lett 49:2216–2220. doi: 10.1016/j.tetlet.2008.02.053
Sharma G, Jyothi Y, Lakshmi P (2006) Efficient room-temperature synthesis of tri- and tetrasubstituted imidazoles catalyzed by \(\text{ ZrCl }_{4}\). Synth Commun 36:2991–3000. doi: 10.1080/00397910600773825
Karimim AR, Alimohammadi Z, Amini MM (2010) Wells-Dawson heteropolyacid supported on silica: a highly efficient catalyst for synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Mol Divers 14:635–641. doi:10.1007/s11030-009-9197-x
Heravi MM, Bakhtiari K, Oskooie HA, Taheri S (2007) Synthesis of 2,4,5-triaryl-imidazoles catalyzed by \(\text{ NiCl }_{2}\cdot \text{6H }_{2}\text{ O }\) under heterogeneous system. J Mol Catal A: Chem 263:279–281. doi:10.1016/j.molcata.2006.08.070
Sangshetti JN, Kokare ND, Kotharkar SA, Shinde DB (2008) Sodium bisulfite as an efficient and inexpensive catalyst for the one-pot synthesis of 2,4,5-triaryl-1\(H\)-imidazoles from benzil or benzoin and aromatic aldehydes. Mont Fur Chem 139:125–127. doi: 10.1007/s00706-007-0766-3
Dake SA, Khedkar MB, Irmale GS, Ukalgaonkar SJ, Thorat VV, Shintre SA, Pawar RP (2012) Sulfated tin oxide: a reusable and highly efficient heterogeneous catalyst for the synthesis of 2,4,5-triaryl-\(1H\)-imidazole derivatives. Synth Commun 42:1509–1520. doi:10.1080/00397911.2010.541744
Parveen A, Ahmed MD, Rafi SK, Shaikh KA, Deshmukh SP, Pawar RP (2007) Efficient synthesis of 2,4,5-triaryl substituted imidazoles under solvent free conditions at room temperature. Arkivoc 16:12–18. doi:10.3998/ark.5550190.0008.g02
Kidwai M, Mothsra P, Bansal V, Goyal R (2006) Efficient elemental iodine catalyzed one-pot synthesis of 2,4,5-triarylimidazoles. Mont Fur Chem 137:1189–1194. doi:10.1007/s00706-006-0518-9
Sangshetti JN, Kakare ND, Kotharkar SA, Shinde DB (2008) Ceric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1\(H\)-imidazoles. J Chem Sci 120:463–467. doi: 10.1007/s12039-008-0072-6
Satyanarayana VSV, Sivakumar A (2011) An efficient and novel one-pot synthesis of 2,4,5-triaryl-1H-imidazoles catalyzed by \(\text{ UO }_{2}(\text{ NO }_{3})_{2}.\text{6H }_{2}\text{ O }\) under heterogeneous conditions. Chem Pap 65:519–526. doi:10.2478/s11696-011-0028-z.ISSN:0366-6352
Siddiqui SA, Narkhede UC, Palimkar SS, Daniel T, Lahoti RJ, Srinivasan KV (2005) Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or \(\alpha \)-hydroxyketone. Tetrahedron 61:3539–3546. doi: 10.1016/j.tet.2005.01.116
Murthy SN, Madhav B, Nageswar YVD (2010) DABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multi-component condensation strategy. Tetrahedron Lett 51:5252–5257. doi:10.1016/j.tetlet.2010.07.128
Surpur MP, Kshirsagar S, Samant SD (2009) Exploitation of the catalytic efficacy of Mg/Al hydrotalcite for the rapid synthesis of 2-aminochromene derivatives via a multicomponent strategy in the presence of microwaves. Tetrahedron Lett 50:719–722. doi:10.1016/j.tetlet.2008.11.114
Sharghi H, Aberi M, Doroodmanda MM (2008) Reusable cobalt(III)-salen complex supported on activated carbon as an efficient heterogeneous catalyst for synthesis of 2-arylbenzimidazole derivatives. Adv Synth Catal 350:2380–2390. doi:10.1002/adsc.200800317
Sharghi H, Aberi M, Doroodmanda MM (2012) One-pot synthesis of 2-arylbenzimidazole, 2-arylbenzothiazole and 2-arylbenzoxazole derivatives using vanadium(IV)-salen complex as homogeneous catalyst and vanadium(IV)-salen complex nanoparticles immobilized onto silica as a heterogeneous nanocatalyst. J Iran Chem Soc 9:189–204. doi:10.1007/s13738-011-0045-4
Sharghi H, Khoshnood A, Doroodmand MM, Khalifeh R (2012) 1,4-Dihydroxyanthraquinone-copper(II) nanoparticles immobilized on silica gel: a highly efficient, copper scavenger and recyclable heterogeneous nanocatalyst for a click approach to the three-component synthesis of 1,2,3-triazole derivatives in water. J Iran Chem Soc 9:231–250. doi:10.1007/s13738-011-0046-3
Sharghi H, Khalifeh R, Mansouri SG, Aberi M, Eskandari MM (2011) Simple, efficient, and applicable route for synthesis of 2-aryl(heteroaryl)-benzimidazoles at room temperature using copper nanoparticles on activated carbon as a reusable heterogeneous catalyst. Catal Lett 141:1845–1850. doi:10.1007/s10562-011-0671-6
Sharghi H, Ebrahimpourmoghaddam S, Doroodmand MM (2013) Facile synthesis of 5-substituted-1\(H\)-tetrazoles and 1-substituted-1\(H\)-tetrazoles catalyzed by recyclable 4\(^{\prime }\)-phenyl-2,2\(^{\prime }\):6\(^{\prime }\),2\(^{\prime \prime }\)-terpyridine copper(II) complex immobilized onto activated multi-walled carbon nanotubes. J Organomet Chem 738:41–48. doi: 10.1016/j.jorganchem.2013.04.013
Sabry DY, Youssef TA, El-Medani SM, Ramadan RM (2003) Reactions of chromium and molybdenum carbonyls with bis-(salicylaldehyde)ethylenediimine schiff-base ligand. J Coord Chem 56:1375–1381. doi:10.1080/00958970310001636471
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharghi, H., Aberi, M. & Doroodmand, M.M. A mild, three-component one-pot synthesis of 2,4,5-trisubstituted imidazoles using Mo(IV) salen complex in homogeneous catalytic system and Mo(IV) salen complex nanoparticles onto silica as a highly active, efficient, and reusable heterogeneous nanocatalyst. Mol Divers 19, 77–85 (2015). https://doi.org/10.1007/s11030-014-9558-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9558-y