Abstract
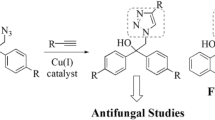
A series of new triazole alcohol antifungals were designed by replacing one of the triazolyl moiety from fluconazole with a distinct 4-amino-3-mercapto-1,2,4-triazole motif, which is found in some antimicrobial agents. The antimicrobial susceptibility testing of target compounds demonstrated that the direct analogs of fluconazole (difluorophenethyl-triazoles) were less active against fungi, while compound 10h containing dichloro substitutions on both phenyl rings of the molecule had potent activity against yeasts including Candida albicans (four strains) and Cryptococcus neoformans (MICs \(=\) 2–8 \({\upmu }\)g/mL). Also, compound 10h was active against Candida parapsilosis, Epidermophyton floccosum, and Trichophyton mentagrophytes, while it showed no activity against Gram-positive and Gram-negative bacteria. Finally, a molecular docking study suggested that compound 10h interacts suitably with lanosterol 14\(\alpha \)-demethylase, which is the key enzyme in ergosterol biosynthesis.







Similar content being viewed by others
References
Plech T, Wujec M, Kosikowska U, Malm A, Kapron B (2012) Studies on the synthesis and antibacterial activity of 3,6-disubstituted 1,2,4-triazolo[3,4-b]1,3,4-thiadiazoles. Eur J Med Chem 47:580–584. doi:10.1016/j.ejmech.2011.10.055
Barbuceanu SF, Saramet G, Almajan GL, Draghici C, Barbuceanu F, Bancescu G (2012) New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenylsulfone moieties: Synthesis, characterization and antimicrobial activity evaluation. Eur J Med Chem 49:417–423. doi:10.1016/j.ejmech.2012.01.031
Shalini K, Kumar N, Drabu S, Sharma PK (2011) Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J Org Chem 7:668–677. doi:10.3762/bjoc.7.79
Xu J, Cao Y, Zhang J, Yu S, Zou Y, Chai X, Wu Q, Zhang D, Jiang Y, Sun Q (2011) Design, synthesis and antifungal activities of novel 1,2,4-triazole derivatives. Eur J Med Chem 46:3142–3148. doi:10.1016/j.ejmech.2011.02.042
Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S (2012) The biology and chemistry of antifungal agents. Bioorg Med Chem 20:5678–5698. doi:10.1016/j.bmc.2012.04.045
Chai X, Zhang J, Cao Y, Zou Y, Wu Q, Zhang D, Jiang Y, Sun Q (2011) Design, synthesis and molecular docking studies of novel triazole as antifungal agent. Eur J Med Chem 46:3167–3176. doi:10.1016/j.ejmech.2011.04.022
Zhang YY, Mi JL, Zhou CH, Zhou XD (2011) Synthesis of novel fluconazoliums and their evaluation for antibacterial and antifungal activities. Eur J Med Chem 46:4391–4402. doi:10.1016/j.ejmech.2011.07.010
Zou Y, Zhao Q, Liao J, Hu H, Yu S, Chai X, Xu M, Wu Q (2012) New triazole derivatives as antifungal agents: synthesis via click reaction, in vitro evaluation and molecular docking studies. Bioorg Med Chem Lett 22:2959–2962. doi:10.1016/j.bmcl.2012.02.042
Jiang Y, Zhang J, Cao Y, Chai X, Zou Y, Wu Q, Zhang D, Jiang Y, Sun Q (2011) Synthesis, in vitro evaluation and molecular docking studies of new triazole derivatives as antifungal agents. Bioorg Med Chem Lett 21:4471–4475. doi:10.1016/j.bmcl.2011.06.008
Williams A, Foye WO, Lemke TL (2002) Foye’s principles of medicinal chemistry. Lippincott Williams and Wilkins publication, New York
Loeffler J, Stevens DA (2003) Antifungal drug resistance. Clin Infect Dis 36:S31–41. doi:10.1086/344658
Collin X, Sauleau A, Coulon J (2003) 1,2,4-Triazolo mercapto and aminonitriles as potent antifungal agents. Bioorg Med Chem Lett 13:2601–2605. doi:10.1016/S0960-894X(03)00378-0
Emami S, Falahati M, Banifatemi A, Amanlou M, Shafiee A (2004) (\(E)\)- and (\(Z)\)-1,2,4-Triazolylchromanone oxime ethers as conformationally constrained antifungals. Bioorg Med Chem 12:3971–3976. doi: 10.1016/j.bmc.2004.06.010
Emami S, Falahati M, Banifatemi A, Shafiee A (2004) Stereoselective synthesis and antifungal activity of (\(Z)\)-trans-3-azolyl-2-methylchromanone oxime ethers. Bioorg Med Chem 12:5881–5889. doi:10.1016/j.bmc.2004.08.030
Emami S, Foroumadi A, Falahati M, Lotfali E, Rajabalian S, Ebrahimi SA, Farahyar S, Shafiee A (2008) 2-Hydroxyphenacyl azoles and related azolium derivatives as antifungal agents. Bioorg Med Chem Lett 18:141–146. doi:10.1016/j.bmcl.2007.10.111
Emami S, Behdad M, Foroumadi A, Falahati M, Lotfali E, Sharifynia S (2009) Design of conformationally constrained azole antifungals: efficient synthesis and antifungal activity of trans-3-imidazolylflavanones. Chem Biol Drug Des 73:388–395. doi:10.1111/j.1747-0285.2009.00797.x
Emami S, Shojapour S, Faramarzi MA, Samadi N, Irannejad H (2013) Synthesis, in vitro antifungal activity and in silico study of 3-(1,2,4-triazol-1-yl)flavanones. Eur J Med Chem 66:480–488. doi:10.1016/j.ejmech.2013.06.008
Borate HB, Maujan SR et al (2010) Fluconazole analogues containing 2\(H\)-1,4-benzothiazin-3(4\(H)\)-one or 2\(H\)-1,4-benzoxazin-3(4\(H)\)-one moieties, a novel class of anti- Candida agents. Bioorg Med Chem Lett 20:722–725. doi:10.1016/j.bmcl.2009.11.071
Bhole RP, Bhusari KP (2011) Synthesis and antitumor activity of (4-hydroxyphenyl)[5-substituted alkyl/aryl)-2-thioxo-1,3,4-thiadiazol-3-yl]methanone and [(3,4-disubstituted)-1,3-thiazol-2-ylidene]-4-hydroxybenzohydrazide. Med Chem Res 20:695–704. doi:10.1007/s00044-010-9371-9
Clinical and Laboratory Standards Institute (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts and filamentous fungi; Approved Standard M27-A3 and M38-A2. Wayne, PA
Upmanyu N, Kumar S, Porwal P, Shah K, Mishra P (2012) Synthesis and evaluation of 4-(substituted)-acetylamino-3-mercapto-5-(4-substituted) phenyl-1,2,4-triazole derivatives as antimicrobial agents. Med Chem Res 21:1967–1976. doi:10.1007/s00044-011-9708-z
Ji D, Lu JR, Lu BW, Xin CW, Mu JB, Li JF, Peng CY, Bao XR (2013) Efficient synthesis and antimicrobial activity of some novel S-\(\beta \)-d-glucosides of 5-aryl-1,2,4-triazole-3-thiones derivatives. Bioorg Med Chem Lett 23:1997–2000. doi: 10.1016/j.bmcl.2013.02.038
Bayrak H, Demirbas A, Demirbas N, Karaoglu SA (2009) Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem 44:4362–4366. doi:10.1016/j.ejmech.2009.05.022
Baro EJ, Finegold SM (2002) Bailey Scott’s diagnostic microbiology, 11th edn. The C. V. Mosby Company, St. Louis, pp 235–236
Podust LM, Poulos TL, Waterman MR (2001) Crystal structure of cytochrome P450 14\(\alpha \)-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc Natl Acad Sci USA 98:3068–3073. doi:10.1073/pnas.061562898
Xiao L, Madison V, Chau AS, Loebenberg D, Palermo RE, McNicholas PM (2004) Three-dimensional models of wild-type and mutated forms of cytochrome P450 14\(\alpha \)-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob Agents Chemother 48:568–574. doi:10.1128/AAC.48.2.568-574.2004
Patel PD, Patel MR, Kocsis B, Kocsis E, Graham SM, Warren AR, Nicholson SM, Billack B, Fronczek FR, Talele TT (2010) Design, synthesis and determination of antifungal activity of 5(6)-substituted benzotriazoles. Eur J Med Chem 45:2214–2222. doi:10.1016/j.ejmech.2010.01.062
Acknowledgments
This work was supported by a grant from the Research Council of Mazandaran University of Medical Sciences, Sari, Iran. This work was related to the Ph.D. thesis of SMH (Faculty of Pharmacy, Mazandaran University of Medical Sciences).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashemi, S.M., Badali, H., Faramarzi, M.A. et al. Novel triazole alcohol antifungals derived from fluconazole: design, synthesis, and biological activity. Mol Divers 19, 15–27 (2015). https://doi.org/10.1007/s11030-014-9548-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9548-0