Abstract
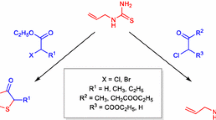
A number of 1,2,4-oxadiazolone and mercapto nitro benzimidazole derivatives containing 1,2,4-oxadiazole and hydantoin moieties have been prepared, and their structures were identified by means of spectral/physical characteristics including X-ray diffraction data.








Similar content being viewed by others
References
Ahn J-M, Boyle NA, MacDonald MT, Janda KD (2002) Peptidomimetics and peptide backbone modifications. Mini Rev Med Chem 2:463–473. doi:10.2174/1389557023405828
Watjen F, Baker R, Engelstoff M, Herbert R, MacLeod A, Knight A, Merchant K, Moseley J, Saunders J, Swain CJ, Wong E, Springer JP (1989) Novel benzodiazepine receptor partial agonists: oxadiazolylimidazobenzodiazepines. J Med Chem 32:2282–2291. doi:10.1021/jm00130a010
Leite ACL, Vieira RF, De Faria AR, Wanderley AG, Afiatpour P, Ximenes ECPA, Srivastava RM, De Oliveira CF, Medeiros MV, Antunes E, Brondani DJ (2000) Synthesis, anti-inflammatory and antimicrobial activities of new 1,2,4-oxadiazoles peptidomimetics. Farmaco 55:719–724. doi:10.1016/S0014-827X(00)00099-9
Bezerra NMM, De Oliveira SP, Srivastava RM, Da Silva JR (2005) Synthesis of 3-aryl-5-decapentyl-1,2,4-oxadiazoles possessing antiinflammatory and antitumor properties. Farmaco 60:955–960. doi:10.1016/j.farmac.2005.08.003
Nicolaides DN, Fylaktakidou KC, Litinas KE, Hadjipavlou-Litina DE (1998) Synthesis and biological evaluation of several coumarin-4-carboxamidoxime and 3-(coumarin-4-yl)-1,2,4-oxadiazole derivatives. Eur J Med Chem 33:715–724. doi:10.1016/S0223-5234(98)80030-5
Diana GD, Volkots DL, Nitz TJ, Bailey TR, Long MA, Vescio N, Aldous S, Pevear DC, Dutko FJ (1994) Oxadiazoles as ester bioisosteric replacements in compounds related to disoxaril. Antirhinovirus activity. J Med Chem 37:2421–2436. doi:10.1021/jm00041a022
Amarasinghe KKD, Evidokimov AG, Xu K, Clark CM, Maier MB, Srivastava A, Colson AO, Gerwe GS, Stake GE, Howard BW, Pokross ME, Gray JL, Peters KG (2006) Design and synthesis of potent, non-peptidic inhibitors of HPTP\(\beta \). Bioorg Med Chem Lett 16:4252–4256. doi: 10.1016/j.bmcl.2006.05.074
Orlek BS, Blaney FE, Brown F, Clark MSG, Hadley MS, Hatcher J, Riley GJ, Rosenberg HE, Wadsworth HJ, Wyman P (1991) Comparison of azabicyclic esters and oxadiazoles as ligands for the muscarinic receptor. J Med Chem 34:2726–2735. doi:10.1021/jm00113a009
Street LJ, Baker R, Book T, Kneen CO, MacLeod AM, Merchant KJ, Showell GA, Saunders J, Herbert RH, Freedman SB, Harley EA (1990) Synthesis and biological activity of 1,2,4-oxadiazole derivatives: highly potent and efficacious agonists for cortical muscarinic receptors. J Med Chem 33:2690–2697. doi:10.1021/jm00172a003
Clitherow JW, Beswick P, Irving WJ, Scopes DIC, Barnes JC, Clapham J, Brown JD, Evans DJ, Hayes AG (1996) Novel 1, 2,4-oxadiazoles as potent and selective histamine H3 receptor antagonists. Bioorg Med Chem Lett 6:833–838. doi:10.1016/0960-894X(96)00122-9
Bolton RE, Coote SJ, Finch H, Lowdon A, Pegg N, Vinader MV (1995) 3-Substituted-1,2,4-oxadiazolin-5-one-a useful amidine precursor and protecting group. Tetrahedron Lett 36:4471–4474. doi:10.1016/0040-4039(95)00755-2
Brown FC (1961) 4-Thiazolidinones. Chem Rev 61:463–521. doi:10.1021/cr60213a002
Labouta IM, Salama HM, Eshba NH, Kader O, El-Chrbini E (1987) Potential anti-microbial: syntheses and in vitro anti-microbial evaluation of some 5-arylazo-thiazolidones and related compounds. Eur J Med Chem 22:485–489. doi:10.1016/0223-5234(87)90287-X
Mohsen A, Omar ME, Salama HM, Eshba NH (1985) Novel thiazolidine-2,4-dione-4-thiosemicarbazone and 4-[(3,4-diaryl-3H-thiazol-2yl)azo]thiazolidin-2-one derivatives-synthesis and evaluation for antimicrobial and anticancer properties. Farmaco 40:49–57
Albuquerque JFC, Rocha JA, Brandao SSF, Lima MCA, Ximenes EA, Galdino SL, Pitta IR, Chantegrel J, Perrissin M, Luu-Duc C (1999) Synthesis and antimicrobial activity of substituted imidazolidinediones and thioxoimidazolidinones. Farmaco 54:77–82. doi:10.1016/S0014-827X(98)00105-0
Swayze EE, Peiris SM, Kucera LS, White EL, Wise DS, Drach JC, Townsend LB (1993) Synthesis of 1-(2-aminopropyl) benzimidazoles, structurally related to the TIBO derivative R82150, with activity against human immunodeficiency virus. Bioorg Med Chem Lett 3:543–546. doi:10.1016/S0960-894X(01)81224-5
Gaur NM, Patil SV, Mourya VK, Wagh SB (2000) Synthesis and SAR of benzimidazole anthelmintics. Indian J Heterocycl Chem 9:227–230
Bhatt AK, Karadia HG, Shah PS, Parmar MP, Patel HD (2004) Synthesis of benzimidazolylphenothiazines and their antibacterial and antifungal activities. Indian J Heterocycl Chem 13:281–282
Mishra RM, Wahab A, Mishra AR (2003) Synthesis and fungicidal activity of some new 2,3-dihydro-4H-benzimidazolo [3,2-b] [1,3]-thiazine-4-ones. Indian J Heterocycl Chem 13:29–32
Sharma P, Mandloi A, Pritmani S (1999) Synthesis of new 2-(substituted benzothiazolylcarbamoyl)benzimidazoles as potential CNS depressants. Indian J Chem 38B:1289–1294
Khan SA, Nandan AM (1997) 2-Substituted benzimidazoles as antiinflammatory and analgesic agents. Indian J Heterocycl Chem 7:55–58
Lazer ES, Matteo MR, Possanza GJ (1987) Benzimidazole derivatives with a typical antiinflammatory activity. J Med Chem 30:726–729. doi:10.1021/jm00387a026
De Almeida MV, Cardoso SH, De Assis JV, De Souza MVN (2007) Synthesis of 2-mercaptobenzothiazole and 2-mercaptobenzimidazole derivatives condensed with carbohydrates as a potential antimicrobial agents. J Sulfur Chem 28:17–22. doi:10.1080/17415990601055291
Mor M, Bordi F, Silva C, Rivara S, Zuliani V, Vacondio F, Rivara M, Barocelli E, Bertoni S, Ballabeni V, Magnanini F, Impicciatore M, Plazzi PV (2004) Synthesis, biological activity, QSAR and QSPR study of 2-aminobenzimidazole derivatives as potent \(\text{ H }_{3}\)-antagonists. Bioorg Med Chem 12:663–674. doi: 10.1016/j.bmc.2003.11.030
Bakhareva EV, Voronkov MG, Sorokin MS, Lopyrev VA, Seredenin SB, Gaidarov GM (1996) Synthesis and neurotropic properties of 2-(carboxymethylthio) derivatives of benzimidazole, benzothiazole and their ammonium salts. Pharm Chem J 30:89–91. doi:10.1007/BF02218873
Hosamani KM, Shingalapur RV (2011) Synthesis of 2-mercaptobenzimidazole derivatives as potential anti-microbial and cytotoxic agents. Arch Pharm Chem Life Sci 344:311–319. doi:10.1002/ardp.200900291
Dürüst Y (2009) Synthesis of some novel 1,3,4-and 1,2,4-thiadiazole derivatives. Phosphorus Sulfur Silicon 184:2923–2935. doi:10.1080/10426500802625453
Dürüst Y, Akcan M, Martiskainen O, Siirola E, Pihlaja K (2008) Synthesis of new thiophene, furan and pyridine substituted 1,2,4,5-oxadiazaboroles. Polyhedron 27:999–1007. doi:10.1016/j.poly.2007.11.043
Dürüst Y, Altuğ C, Kılıç F (2007) Thiophene-substituted 1,2,4-oxadiazoles and oxadiazines. Phosphorus Sulfur Silicon 182:299–313. doi:10.1080/10426500600919124
Dürüst N, Dürüst Y, Meriç İ (2002) Acid–base equilibria of some N-substituted thiophene-2-carboxamidoximes in non-aqueous media. Turk J Chem 26:833–838
Ağırbaş H, Sümengen D, Dürüst Y, Dürüst N (1992) The reaction of amidoximes with chloroacetyl chloride. Synth Commun 22:209–217. doi:10.1080/00397919208021295
Ağırbaş H, Sümengen D, Dürüst Y (1992) Synthesis and methylation of some 1,2,4-thiadiazole-5-thiones. Phosphorus Sulfur Silicon 66:321–324. doi:10.1080/10426509208038363
Katritzky AR, Lagowski JM (1963) Adv Heterocycl Chem 1:311–333. doi:10.1016/S0065-2725(08)60528-0
Lacasse G, Muchowski JM (1973) 5-Membered heterocyclic thiones. 1,2,4-thiadiazole-3-thiolates and 1,2,4-thiadiazole-5-thione. Can J Chem 51:2353–2356. doi:10.1139/v73-352
Acknowledgments
Abant İzzet Baysal University, Directorate of Research Projects Commission (BAP Grant No. 2011.03.03.442) is gratefully acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dürüst, Y., Gözlükaya, Ö., Özer, G. et al. Synthesis and crystal structure of new heterocycles containing 1,2,4-oxadiazole, 1,2,4-oxadiazolone (thione), hydantoin, and mercaptobenzimidazole units. Mol Divers 18, 545–558 (2014). https://doi.org/10.1007/s11030-014-9525-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9525-7