Summary
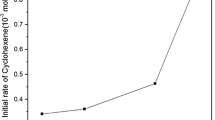
Ruthenium byproducts from ring-closing metathesis reactions can be removed by refluxing the crude reaction mixture with resin-bound triphenylphosphine oxide (TPPO) in toluene or by stirring with dimethyl sulfoxide (DMSO) and silica gel at room temperature. Residual levels of ruthenium can be achieved that are as low as 0.04 μg per 5 mg of product when a combination of TPPO, DMSO, and silica gel is used. The polymer-bound TPPO retained its efficiency after being recycled six times.
Similar content being viewed by others
Abbreviations
- DMSO:
-
dimethylsulfoxide
- equiv:
-
equivalent
- h:
-
hour(s)
- ICP-MS:
-
inductive-coupled plasma mass spectrometry
- IR:
-
infrared spectroscopy
- MAS:
-
magic angle spinning
- NMR:
-
nuclear magnetic resonance spectroscopy
- rt:
-
room temperature
- SiO2:
-
silica gel
- Temp:
-
temperature
- TPP:
-
triphenylphosphine
- TPPO:
-
triphenylphosphine oxide
References
Grubbs, R.H. and Chang, S., Recent advances in the Olefin metathesis and its application in organic synthesis, Tetrahedron, 54 (1998) 4413–4450.
Trnka, T.M. and Grubbs, R.H., The development of L2 * Ru = CHR olefin metathesis catalysts: An organometallic success story, Acc. Chem. Res., 34 (2001) 18–29.
Fürstner, A., Olefin metathesis and beyond, Angew. Chem., 39 (2000) 3012–3043.
Schuster, M. and Blechert, S., Olefin metathesis in organic chemistry, Angew. Chem., Int. Ed. Engl., 36 (1997) 2036–2056.
Schrock, R.R., Olefin Metathesis by Well-Defined Complexes of Molybdenum and Tungsten. Topics in Organometallic Chemistry. Alkene Methathesis in Organic Synthesis, Springer, Berlin, 1998, pp. 1–36.
Cho, J.H. and Kim, B.M., An efficient method for removal of ruthenium byproducts from olefin metathesis reactions, Org. Lett., 5 (2003) 531–533.
Maynard, H.D. and Grubbs, R.H., Purification technique for the removal of ruthenium from olefin metathesis reaction products, Tetrahedron Lett., 40 (1999) 4137–4140.
Paquette, L.A., Schloss, J.D., Efremov, I., Fabris, F., Gallou, F., Mendez-Andino, J. and Yang, J., A convenient method for removing all highly-colored byproducts generated during olefin metathesis reactions, Org. Lett., 2 (2000) 1259–1261.
Ahn, Y.M., Yang, K., and Georg, G.I., A convenient method for the efficient removal of ruthenium by-products generated during olefin metathesis reactions using triphenylphosphine oxide or dimethyl sulfoxide, Org. Lett., 3 (2001) 1411–1413.
Regen, S.L. and Lee, D.P., Solid phase phosphorus reagents. conversion of alcohols to. Alkyl Chlorides, J. Org. Chem., 40 (1975) 1669–1670. Polymer-supported TPP, cross-linked with 2% DVB, ∼3mmol P/g resin, was purchased from Aldrich.
Pouchert, C.J., The Aldrich Library of FT-IR Spectra, 1st Ed.; Vol 2. TPP: FT-IR 1(2), 539D: TPPO: FT-IR 1(2), 559A.
The 31P resonance for TPP at −6.7 ppm disappeared and the 31P resonance for TPPO at 29 ppm appeared. Rentsch, D.; Hany, R.; Barthelemy, S., and Steinauer, R., Quantitative Determination of Loadings and Oxidation Products of Polystyrene-Bound Phosphines Using 31P MAS NMR, J. Comb. Chem., 5 (2003) 610–616.
Rentsch, D., Hany, R., Barthelemy, S. and Steinauer, R., Improved reproducibility of chemical reactions on purified polystyrene resins monitored by 31P MAS NMR, Tetrahedron Lett., 44 (2003) 6987– 6990.
Evidence that DMSO might destroy the ruthenium catalyst comes from Liras' work, who reported that a molecule bearing the sulfoxide functionality required the full equivalency of the Grubbs catalyst to drive the RCM reaction to completion. Liras, S., Davoren, J.E., and Bordner, J., An approach to the skeleton of the securinega alkaloids. The total synthesis of (±)-Securinine, Org. Lett., 3 (2001) 703–706.
Procedure for entries 2–5: To a solution of diethyl diallylmalonate (0.17 mmole) dissolved in CH2Cl2 (40 mL) was added catalyst 1 (5 mol%). The mixture was stirred for 2 h and then the solvent was removed under reduced pressure. To the crude products toluene (5 mL), resin-bound TPPO (50 equiv, 145 mg) and DMSO (0.03 mL) were added, followed by heating for 6 h. The mixture was loaded on 1.8 g of silica gel for flash column chromatography and was eluted with 20% EtOAc in hexanes. Procedure for entries 6–10: To the crude products in toluene (5 mL), DMSO (0.03 mL), resin-bound TPPO (50 equiv, 45 mg) and silica gel (1.8 g) were added, followed by heating for 6 h. The mixture was filtered through a cotton-blocked pipette using 20% EtOAc in hexane.
The catalyst batch used in the experiments in Tables 1–3 was purchased from Strem. The additional experiments were carried out with a batch of catalyst purchased from Aldrich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haack, K., Ahn, Y.M. & Georg, G.I. A convenient method to remove ruthenium byproducts from olefin metathesis reactions using polymer-bound triphenylphosphine oxide (TPPO). Mol Divers 9, 301–303 (2005). https://doi.org/10.1007/s11030-005-2480-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11030-005-2480-6