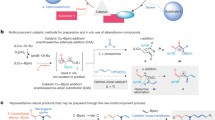
Abstract
Parallel combinatorial synthesis in solution using immobilized reagents, catalysts, and scavengers has emerged as a powerful technique for the preparation of diverse libraries of compounds. This technique has only recently been applied to the synthesis of large-ring compounds. In this comprehensive review several strategies are presented and discussed, including Pd-catalyzed allylic alkylation, Stille-coupling, macrolactonization and macrolactamization using solid supported reagents and catalysts. In several cases site isolation has allowed operation of these macrocyclization reactions in concentrated solution (pseudo-dilution effect).
Similar content being viewed by others
Abbreviations
- DBU:
-
1,8-diazabicyclo[5.4.0]undec-7ene
- DEAD:
-
Di(ethyl)azodicarboxylate
- DIAD:
-
Di(isopropyl)azodicarb oxylate
- DMAP:
-
N,N-Dimethyl-4-aminopyridine
- dppe:
-
Bis(diphenylphosphino)ethane
- dppf:
-
1,1′-Bis(diphenylphosphino) ferrocene
- Eq.:
-
Equivalent
- HOBT:
-
1-Hydroxybenzotriazole
- PDBU:
-
polystyrene-supported 1, 8-diazabicyclo[5.4.0]undec-7ene
- PEG:
-
Polyethylenglykol
- PS:
-
Polystyrene
References
Roxburgh, C.J., The syntheses of large-ring compounds, Tetrahedron, 51 (1995) 9767–9822.
Lambert, J.N., Mitchell, J.P. and Roberts, K.D., The synthesis of cyclic peptides, J. Chem. Soc., Perkin Trans., 1 (2001) 471–484.
Li, P. and Roller, P.P, Cyclization strategies in peptide derived drug design, Curr. Top. Med. Chem., 2 (2002) 325–341.
Masamune, S., Bates, G.S. and Corcoran, J.W., Makrolide. neuere fortschritte ihrer chemie und biochemie, Angew. Chem., 89 (1977) 602–624.
Rousseau, G., Medium ring lactones, Tetrahedron, 51 (1995) 2777–2849.
Nicolaou, K.C., Synthesis of macrolides, Tetrahedron, 33 (1977) 683–710.
Golebiowski, A., Klopfenstein, S.R. and Portlock, D.E., Lead compounds discovered from libraries, Curr. Opin. Chem. Biol., 5 (2001) 273–284.
Golebiowski, A., Klopfenstein, S.R. and Portlock, D.E., Lead compounds discovered from libraries: Part 2, Curr. Opin. Chem. Biol., 7 (2003) 308–325.
Dolle, R.E., Comprehensive survey of combinatorial library synthesis: 2002, J. Comb. Chem., 5 (2003) 693–753.
Dolle, R.E., Comprehensive survey of combinatorial library synthesis: 2001, J. Comb. Chem., 4 (2002) 369–418.
Dolle, R.E., Comprehensive survey of combinatorial library synthesis: 2000, J. Comb. Chem., 3 (2001) 477–517.
Dolle, R.E., Comprehensive survey of combinatorial library synthesis: 1999, J. Comb. Chem., 2 (2000) 383–433.
Dolle, R.E., Comprehensive survey of combinatorial library synthesis: 1998, J. Comb. Chem., 1 (1999) 235–282.
Dolle, R.E., Comprehensive survey of chemical libraries yielding enzyme inhibitors, receptor agonists and antagonists, and other biologically active agents: 1992 through 1997, Mol. Div., 3 (1998) 199–233.
Dolle, R.E., Comprehensive survey of combinatorial libraries with undisclosed biological activity: 1992 through 1997, Mol. Div., 3 (1998) 233–256.
Nicolaou, K.C., Winssinger, N., Pastor, J., Ninkovic, S., Sarabia, F., He, Y., Vourloumis, D., Yang, Z., Li, T., Giannakakou, P. and Hamel, E., Synthesis of epothilones A and B in solid and solution phase, Nature, 387 (1997) 268–272.
Nicoloau, K.C., Winssinger, N., Pastor, J. and Murphy, F., Solid Phase Synthesis of Macrocyclic Systems by a Cyclorelease Strategy. Application of the Stille Coupling to a Synthesis of (S)-Zearalenone, Angew. Chem. Int. Ed., 37 (1998) 2534–2537.
Kiselyov, A.S., Eisenberg, S. and Luo, Y., Solid support synthesis of 14-membered macrocycles via SNAr methodology on acrylate resin, Tetrahedron Lett., 40 (1999) 2465–2468.
Bourne, G.T., Golding, S.W., McGeary, R.P., Meeutermans, W.D.F., Jones, A., Marshall, G.R., Alewood, P.F. and Smythe M.L., The development and application of a novel safety-catch linker for BOC-based assembly of libraries of cyclic peptides, J. Org. Chem., 66 (2001) 7706–7713.
Seitz, O., Die cyclisierende ablösung, Nachr. Chem., 49 (2001) 912–916.
Kirschning, A., Monenschein, H. and Wittenberg, R., Functionalized polymers—emerging versatile tools for solution-phase chemistry and automated parallel synthesis, Angew. Chem. Int. Ed., 40 (2001) 650–679.
Ley, S.V., Baxendale, I.R., Bream, R.N., Jackson, P.S., Leach, A.G., Longbottom, D.A., Nesi, M., Scott, J.S., Storer, R.I. and Taylor, S.J., Multi-step organic synthesis using solid-supported reagents and scavengers: A new paradigm in chemical library synthesis, J. Chem. Soc., Perkin Trans. 1 (2000) 3815–4195.
Parlow, J.J., Devraj, R.V. and South, M.S., Solution-phase chemical library synthesis using polymer-assisted purification techniques, Curr. Opin. Chem. Biol., 3 (1999) 320–336.
Kaldor, S.W. and Siegel, M.G., Combinatorial chemistry using polymer-supported reagents, Curr. Opin. Chem. Biol., 1 (1997) 101–106.
McNamara, C.A., Dixon, M.J. and Bradley, M., Recoverable catalysts and reagents using recyclable polystyrene-based supports, Chem. Rev., 102 (2002) 3275–3300.
Clapham, B., Reger, T.S. and Janda, K.D., Polymer-supported catalysis in synthetic organic chemistry, Tetrahedron, 57 (2001) 4637–4662.
Scott, L.T. and Naples, J.O., Macrolide synthesis by the polystyrene-mediated lactonization of ω-hydroxycarboxylic acids, Synthesis, (1976) 738–739.
Regen, S.L. and Kimura, Y., Triphase catalytic cyclization. Efficiacious macrolide synthesis, J. Am. Chem. Soc., 104 (1982) 2064–2065.
Regen, S.L. and Kimura, Y., High dilution via solid–liquid phase transfer catalysis. A practical approach to the synthesis of macrolides, J. Org. Chem., 48 (1983) 1533–1534.
Hodge, P., Ji-Long, J., Owen, G.J. and Houghton, M.P., Reactions of polymer-supported ω-bromoalkylcarboxylates: Formation of lactones versus oligomerization, Polymer, 37 (1996) 505–5067.
Tomoi, M., Watanabe, T., Suzuki, T. and Kakiuchi, H., Macrolide synthesis from ω-bromocarboxylic acids using polymer-supported 1,8-diazabicyclo[5.4.0]undec-7-ene, Macromol. Chem., 186 (1985) 2473–2481.
Amos, R.A., Emblidge, R.W. and Havens, N., Esterification using a polymer-supported phosphine reagent, J. Org. Chem., 48 (1983) 3598–3600.
Herb, C. and Maier, M.E., A Formal Total Synthesis of the Salicylhalamides, J. Org. Chem., 68 (2003) 8129–8135.
Boden, E.P. and Keck, G.E., Proton-transfer steps in Steglich esterification: A very practical new method for macrolactonization, J. Org. Chem., 50 (1985) 2394–2395.
Keck, G.E., Sanchez, C. and Wager, C.A., Macrolactonization of hydroxyl acids using a polymer bound carbodiimide, Tetrahedron Lett., 41 (2000) 8673–8676.
Storer, R.I., Takemoto, T., Jackson, P.S. and Ley, S.V., A total synthesis of epothilones using solid-supported reagents and scavengers, Angew. Chem. Int. Ed., 42 (2003) 2521–2525.
Inanaga, J., Hirata, K., Saeki, H., Katsuki, H. and Yamaguchi, M., A rapid esterificaion by means of mixed anhydride and its application to large-ring lactonization, Bull. Chem. Soc. Jpn., 52 (1979) 1989–1973.
Huang, W. and Kalivretenos, A.G., Synthesis of medium ring lactams Via cyclization reactions using polymer bound HOBT as catalyst, Tetrahedron Lett., 36 (1996) 9113–9116.
Trost, B.M. and Warner, R.W., Makrocyclization via an isomerization reaction at high concentrations promoted by palladium templates, J. Am. Chem. Soc., 104 (1982) 6112–6114.
Trost, B.M. and Warner, R.W., Macrolide formation via an isomerization reaction. An unusual dependence on nucleophile, J. Am. Chem. Soc., 105 (1983) 5940–5942.
Trost, B.M., Cyclizations via palladium-catalyzed allylic alkylations, Angew. Chem. Int. Ed. Engl., 28 (1999) 1173–1192.
Fürstner, A. and Weintritt, H., Total synthesis of roseophilin, J. Am. Chem. Soc., 120 (1998) 2817–2825.
Stille, J.K., Su, H., Hill, D.H., Schneider, P., Tanaka, M., Morrison, D.L. and Hegedus, L.S., Synthesis of large-ring keto lactones by the homogeneous and polymer-supported palladium-catalyzed carbonylative coupling of esters having vinyl triflate and vinylstannane termini, Organometallics, 10 (1991) 1993–2000.
Barrett, A.G.M., Hennessy, A.J., Le Vezouet, R., Procopiou, P.A., Seale, P.W., Stefaniak, S., Upton, R.J., White, A.J.P. and Williams, D.J., Synthesis of diverse macrocyclic peptidomimetics utilizing ring-closing metathesis and solid-phase synthesis, J. Org. Chem, 69 (2004) 1028–1037.
Annis, I., Chen, L. and Barany, G., Novel solid-phase reagents for facile formation of intramolecular disulfide bridges in peptides under mild conditions, J. Am. Chem. Soc., 120 (1998) 7226–7238.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonthier, E., Breinbauer, R. Solid-supported reagents and catalysts for the preparation of large ring compounds. Mol Divers 9, 51–62 (2005). https://doi.org/10.1007/s11030-005-1308-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11030-005-1308-8