Abstract
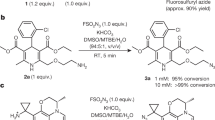
Fluorous tagging strategy is applied to solution-phase parallel synthesis of a library containing hydantoin and thiohydantoin analogs. Two perfluoroalkyl (Rf)-tagged α-amino esters each react with six aromatic aldehydes under reductive amination conditions. Twelve amino esters then each react with 10 isocyanates and isothiocyanates in parallel. The resulting 120 ureas and thioureas undergo spontaneous cyclization to form the corresponding hydantoins and thiohydantoins. The intermediate and final product purifications are performed with solid-phase extraction (SPE) over FluoroFlashTM cartridges, no chromatography is required. Using standard instruments and straightforward SPE technique, one chemist accomplished the 120-member library synthesis in less than five working days, including starting material synthesis and product analysis.
Similar content being viewed by others
Abbreviations
- Rf:
-
perfluoroalkyl group
- SPE:
-
solid-phase extraction
- DIC:
-
1,3-diisopropylcarbodiimide
- HOBT:
-
1-hydroxybenzotriazole
- Fmoc:
-
9-fluorenylmethoxycarbonyl
- Boc:
-
t-butoxycarbonyl
- THF:
-
tetrahydrofuran
- DMAP:
-
4-dimethylaminopyridine
- DMF:
-
N,N-dimethylformamide
- HPLC:
-
high-performance liquid chromatography
- TLC:
-
thin-layer chromatography
- MS:
-
mass spectrometry
- NMR:
-
nuclear magnetic resonance spectroscopy
- LC-MS:
-
liquid chromatography-mass spectrometry
- FTI:
-
Fluorous Technologies, Inc.
References
Zhang, W., Fluorous technologies for solution-phase high-throughput organic synthesis, Tetrahedron, 59 (2003) 4475–4489.
Gladysz, J.A. and Curran, D.P., Fluorous chemistry: From biphasic catalysis to a parallel chemical universe and beyond, Tetrahedron, 58 (2002) 3823–3825.
Curran, D.P., In Stoddard, F., Reinhoudt, D., Shibasaki, M., Ed. Stimulating Concepts in Chemistry, Wiley-VCH, New York, 2000, pp. 25–37.
Curran, D.P., Strategy-level separations in organic synthesis: From planning to practice, Angew. Chem. Int. Ed., 37 (1998) 1175–1196.
Curran, D.P., In Gladysz, J.A., Hovath, I., Curran, D.P., Ed., The Handbook of Fluorous Chemistry, Wiley-VCH, Weinheim, 2004.
Curran, D.P., Fluorous reverse phase silica gel. a new tool for preparative separations in synthetic organic and organofluorine chemistry, Synlett (2001) 1488–1496.
Curran, D.P. and Oderaotoshi, Y., Thiol additions to acrylates by fluorous mixture synthesis: Relative control of elution order in demixing by the fluorous tag and the thiol substituent, Tetrahedron, 57 (2001) 5243–5253.
Dandapani, S. and Curran, D.P., Fluorous Mitsunobu reagents and reactions, Tetrahedron, 58 (2002) 3855–3864.
Dobbs, A.P. and McGregor-Johnson, C., Synthesis of fluorous azodicarboxylates: Towards cleaner Mitsunobu reactions, Tetrahedron Lett., 43 (2002) 2807–2810.
Crich, D. and Neelamkavil, S., The fluorous Swern and Corey-Kim reactions: Scope and mechanism, Tetrahedron, 58 (2002) 3865-3870.
Lindsley, C.W., Zhao, Z., Newton, R.C., Leister, W. and Strauss, K.A., A general protocol for solution-phase parallel synthesis, Tetrahedron Lett., 43 (2002) 4467–4470.
Werner, S. and Curran, D.P., Fluorous dienophiles are powerful diene scavengers in Diels-Alder reactions, Org. Lett., 5 (2003) 3293–3296.
Zhang, W., Chen, C.H.-T. and Nagashima, T., Fluorous electrophilic scavengers for solution-phase parallel synthesis, Tetrahedron Lett., 44 (2003) 2065–2068.
Zhang, W., Curran, D.P. and Chen, C.H.-T., Use of fluorous silica gel to separate fluorous thiol quenching derivatives in solution-phase parallel synthesis, Tetrahedron, 58 (2002) 3871–3875.
Lindsley, C.W., Zhao, Z. and Leister, W., Fluorous-tethered quenching reagents for solution phase parallel synthesis, Tetrahedron Lett., 43 (2002) 4225–4228.
Lindsley, C.W., Zhou, Z., Leister, W.H. and Strauss, K.A., Fluorous-tethered amine bases for organic and parallel synthesis: Scope and limitations, Tetrahedron Lett., 44 (2003), 3619–3623.
Curran, D.P., Amatore, M., Guthrie, D., Campbell, M., Go, E. and Luo, Z., Synthesis and reactions of fluorous carbobenzyloxy (F-cbz) derivatives of α-amino acids, J. Org. Chem., 68 (2003) 4643-4647.
de Visser, P.C., van Helden, M., Filippov, D.V., van der Marel, G., Drijfhout, J.W., van Boom, J.H., Noort, D. and Overkleet, H.S., A novel, base-labile fluorous amine protecting group: Synthesis and use as a tag in the purification of synthetic peptide, Tetrahedron Lett., 44 (2003) 9013–9016.
Read, R. and Zhang, C. Synthesis of fluorous acetal derivatives of aldehydes and ketones, Tetrahedron Lett., 44 (2003) 7045–7047.
Luo, Z.Y., Williams, J., Read, R.W. and Curran, D.P, Fluorous Boc (F-Boc) carbamates: New amine protecting groups for use in fluorous synthesis, J. Org. Chem., 66 (2001) 4261–4266.
Rover, S. and Wipf, P., Synthesis and applications of fluorous silyl protecting groups with improved acid stability, Tetrahedron Lett., 40 (1999) 5667–5670.
Zhang, W., Fluorous synthesis of disubstituted pyrimidines, Org. Lett., 5 (2003) 1011–1013.
Chen, C.H.-T. and Zhang, W., FluoMar, A fluorous version of the Marshall resin for solution-phase library synthesis, Org. Lett., 5 (2003) 1015–1017.
Zhang, Q., Lu, H., Richard, C. and Curran, D.P., Fluorous mixture synthesis of stereoisomer libraries: Total syntheses of (+)-murisolin and fifteen diastereoisomers, J. Am. Chem. Soc., 126 (2004) 36-37.
Zhang, W., Luo, Z.Y., Chen, C.H.-T. and Curran, D.P., Solution-phase preparation of a 560-compound library of individually pure mappicine analogs by fluorous mixture synthesis, J. Am. Chem. Soc., 124 (2002) 10443–10450.
Zhang, Q., Rivkin, A. and Curran, D.P., Quasiracemic synthesis: Concepts and implementation with a fluorous tagging strategy to make both enantiomers of pyridovericin and mappicine, J. Am. Chem. Soc., 124 (2002) 5774–5781.
Curran, D.P. and Furukawa, T., Simultaneous preparation of four truncated discodermolide analogs by fluorous mixture synthesis, Org. Lett., 4 (2002) 2233–2235.
Luo, Z.Y., Zhang, Q.S., Oderaotoshi, Y. and Curran, D.P., Fluorous mixture synthesis: A fluorous-tagging strategy for the synthesis and separation of mixtures of organic compounds, Science, 291 (2001) 1766–1769.
Mazoni, L., Rapid synthesis of oligosaccharides using an anomeric fluorous silyl protecting group, Chem. Commun. (2003) 2930–2931.
Filippov, D.V., van Zoelen, D.J., Oldfield, S.P., van der Marel, G.A., Overkleeft, H.S., Drijfhoutb, J.W. and van Boom, J.H., Use of benzyloxycarbonyl (z)-based fluorophilic tagging reagents in the purification of synthetic peptides, Tetrahedron Lett., 43 (2002) 7809-7812.
Palmacci, E.R., Hewitt, M.C. and Seeberger, P.H., ‘Cap-tag’-novel methods for the rapid purification of oligosaccharides prepared by automated solid-phase synthesis, Angew. Chem. Int. Ed., 40 (2001) 4433–4437.
Nakajima, M., Itoi, K., Takamatsu, Y., Kinoshita, T., Okazaki, T., Kawakubo, K., Shindo, M., Honma, T., Tohjigamori, M. and Haneishi, T., Hydantocidin: A new compound with herbicidal activity from Streptomyces hygroscopicus. J. Antibiot., 44 (1991) 293–300.
Haruyama, H., Takayama, T., Kinoshita, T., Kondo, M., Nakajima, M. and Haneishi, T., Structural elucidation and solution conformation of the novel herbicide hydantocidin, J. Chem. Soc. Perkin Trans. I (1991) 1637–1640.
Harrington, M.P. and Jung, M.E., Stereoselective bromination of β-ribofuranosyl amide. Enantioselective synthesis of (+)-hydantocidin, Tetrahedron Lett., 35 (1994) 5145–5148.
Hollenbeak, K.H. and Schmitz, F.J., Aplysinopsin: Antineoplastic tryptophan derivative from the marine sponge Verongia spengelii, Lloydia, 40 (1977) 479–481.
Kazlauskas, R., Murphy, P.T., Quinn, R.J. and Wells, R.J., Aplysinopsin, A new tryptophan derivative from a sponge, Tetrahedron Lett., 18 (1977) 61–64.
Jakse, R., Kroselj, V., Recnik, S., Sorsak, G., Svete, J., Stanovnik, B. and Gradadolnik, S.G., Stereoselective synthesis of 5-[(Z)-heteroarylmethylidene] substituted hydantoins and thiohydantoins as aplysinopsin, Z. Naturforsch, 57b (2002) 453–459.
Ware, E., The chemistry of the hydantoins, Chem. Rev., 46 (1950) 403–470.
Struck, R.F., Kirk, M.C., Rice, L.S. and Suling, W.J., Isolation, synthesis and antitumor evaluation of spirohydantoin aziridine, a mutagenic metabolite of spirohydantoin mustard, J. Med. Chem., 29 (1986) 1319–1321.
Matsukura, M., Daiku, Y., Ueda, K., Tanaka, S., Igarashi, T. and Minami, N., Synthesis and antiarrhythmic activity of 2,2-dialkyl-1′-(N-substituted aminoalkyl)-spiro-[chroman-4,4′-imidazolidine]-2′,5′-diones, Chem. Pharm. Bull., 40 (1992) 1823–1827.
Brouillete, W.J., Jestkov, V.P., Brown, M.L., Akhtar, M.S., DeLorey, T.M. and Brown, G.B., Bicyclic hydantoins with a bridgehead nitrogen. comparison of anticonvulsant activities with binding to the neuronal voltage-dependent sodium channel, J. Med. Chem., 37 (1994) 3289–3293.
Nefzi, A., Giulianotti, M., Truong, L., Rattan, S., Ostresh, J.M. and Houghten, R.A., Solid-phase synthesis of linear ureas tethered to hydantoins and thiohydantoins, J. Comb. Chem., 4 (2002) 175–178, references cited therein.
Mizuno, T., Kino, T., Ito, T. and Miyata, T., Synthesis of aromatic urea herbicides by the selenium-assisted carbonylation using carbon monoxide with sulfur, Synth. Commun., 30 (2000) 1675-1688.
Mio, S., Ichinose, R., Goto, K., Sugai, S. and Sato, S., Synthetic studies on (+)-hydantocidin (1): A total synthesis of (+)-hydantocidin, a new herbicidal metabolite from microorganism, Tetrahedron, 47 (1991) 2111–2120.
Mio, S., Shiraishi, M., Sugai, S., Haruyama, H. and Sato, S., Synthetic studies on (+)-hydantocidin (2): aldol addition approaches toward the stereoisomers of (+)-hydantocidin, Tetrahedron, 47 (1991) 2121–2132.
Mio, S., Kumagawa, Y. and Sugai, S., Synthetic studies on (+)-hydantocidin (3): A new synthetic method for construction of the spiro-hydantoin ring at the anomeric position of D-ribofuranose, Tetrahedron, 47 (1991) 2133–2144.
Chemla, P., Stereoselective synthesis of (+)-hydantocidin, Tetrahedron Lett., 34 (1993) 7391–7394.
Fischer, H.-P., Buser, H.-P., Chemla, P., Huxley, P., Lutz, W., Mirza, S., Tombo, G.M.R., van Lommen, G. and Sipido, V., Synthesis and chirality of novel heterocyclic compounds designed for crop protection, Bull. Soc. Chim. Belg., 103 (1994) 565–581.
Sano, H. and Sugai, S., Synthesis of (±)-carbocyclic analogue of spirohydantoin nucleoside, Tetrahedron, 51 (1995) 4635–4646.
Mui, M. and Ganesan, A., Solution-phase synthesis of a combinatorial thiohydantoin library, J. Org. Chem., 62 (1997) 3230–3235.
Sosa, A.C.B., Yakushijin, K. and Horne, D.A., Synthesis of axinohydantoins, J. Org. Chem., 67 (2002) 4498–4500.
Lamothe, M., Lannuzel, M. and Perez, M., Solid-phase preparation of hydantoins through a new cyclization’cleavage step, J. Comb. Chem., 4 (2002) 73–78.
Kita, R., Svec, F. and Frechet, J.M.J., Hydrophilic polymer supports for solid-phase synthesis: Preparation of poly(ethylene glycol) methacrylate polymer beads using “classical” suspension polymerization in aqueous medium and their application in the solid-phase synthesis of hydantoins, J. Comb. Chem., 3 (2001) 564-571.
Charton, J., Delarue, S., Vendeville, S., Debreu-Fontaine, M.-A., Mizzi, S. and Sergheraert, C., Convenient synthesis of tetrahydroisoquinoline-hydantoins, Tetrahedron Lett., 42 (2001) 7559–7561.
Park, K.-H., Ehrler, J., Spoerri, H. and Kurt, M.J., Preparation of a 990-member chemical compound library of hydantoin- and isoxazoline-containing heterocycles using multipin technology, J. Comb. Chem., 3 (2001) 171–176.
Dorwald, F.Z., Organic Synthesis on Solid Phase; Wiley-VCH, Weinheim, 2000, pp. 363–366, references cited therein.
Bräse, S. and Dahmen, S., In Nicolaou, K.C., Hanko, R., Hartwig, W., Ed. Handbook of Combinatorial Chemistry, Wiley-VCH, Weinheim, 2002, Vol. 1, pp. 59–169.
DeWitt, S.H., Kiely, J.S., Stankovic, C.J., Schroeder, M.C., Cody, D.M. R. and Pavia, M.R., “Diversomers”: An approach to nonpeptide, nonoligomeric chemical diversity, Proc. Natl. Acad. Sci., 90 (1993) 6909–6913.
Zhang, W. and Lu, Y., Fluorous synthesis of hydantoins and thiohydantoins, Org. Lett., 5 (2003), 2555–2558.
Zhang, W., Lu, Y. and Chen, C.H.-T., Combination of microwave reactions with fluorous separations in the palladium-catalyzed synthesis of aryl sulfides, Mol. Divers., 7 (2003) 199–202.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Zhang, W. Fluorous parallel synthesis of a hydantoin/thiohydantoin library. Mol Divers 9, 91–98 (2005). https://doi.org/10.1007/s11030-005-1293-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11030-005-1293-y