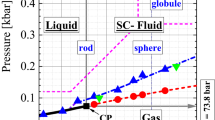
Temperature dependences of the isobaric heat capacity of stable and supercooled liquid carbon, which were obtained by processing thermograms of spontaneous cooling of spherical samples (levitation experiment), were determined. Using the thermodynamic analogy of the properties of the elements of the carbon subgroup of the Periodic Table for carbon, surface tension at the crystal–liquid interface, the size and the work of formation of the critical nucleus of the solid phase in a supercooled liquid, and the heat of fusion were found.




Similar content being viewed by others
References
P.-F. Paradis, T. Ishikavwa, and S. Yoda, “Noncontact measurements of thermophysical properties of molybdenum at high temperatures,” Int. J. Thermophys., 23, No. 2, March, 555–568 (2002).
P.-F. Paradis, T. Ishikavwa, and S. Yoda, “Thermophysical property measurements of supercooled and liquid rhodium,” Int. J. Thermophys., 4, July, 1121–1136 (2003).
W. K. Rhim and K. Ohsaka, “Thermophysical properties measurement of molten silicon by high-temperature electrostatic levitator: density, volume expansion, specific heat capacity, emissivity, surface tension and viscosity,” J. Crystal Growth, 208, 313–321 (2000).
W. K. Rhim and T. Ishikawa, “Thermophysical properties of molten germanium measured by a high-temperature eleckrostatic levitator,” Int. J. Thermophys., 21, No. 2, 429–443 (2000).
C. Ronchi, R. Beukers, H. Heinz, et al., “Graphite melting under laser pulser heating,” Int. J. Thermophys., 139, No. 1, 107–129 (1992).
E. I. Asinovsky, A. V. Kirillin, and A. V. Kostanovsky, “On the question of the phase diagram of carbon near the solid-liquid-vapor triple point,” Teplofiz. Vys. Temp., 35, No. 5, 716–721 (1997).
B. B. Khlevnoi, “Experimental study of the metrological characteristics of high-temperature black bodies based on the phase transitions of the Ir–C and Re–C eutectics,” Metrologiya, No. 2, 18–28 (2001).
A. V. Kostanovsky, M. E. Kostanovskaya, and M. G. Zeodinov, “Features of determining the thermal conductivity of graphite at temperatures of 3000–3300 K,” Izmer. Tekhn., No. 5, 37–42 (2011).
A. E. Sheindlin (ed.), Radiative Properties of Solid Materials, Energiya, Moscow (1974).
V. P. Sosedov (ed.), Properties of Carbon-Based Construction Materials, Metallurgiya, Moscow (1975).
N. S. Rasor and J. D. McClelland, “Thermal properties of graphite, molybdenum and tantalum to their destruction temperatures,” J. Phys. Chem. Solids, 15, 17–26 (1960).
J. H. Lundell and R. R. Dickey, “Ablation of graphitic materials in the sublimation regime,” AIAAJ, 13, No. 8, 1079–1085 (1975).
Y. S. Touloukian (ed.), Thermophysical Properties of High Temperature Solid Materials, Macmillan Co., N.Y., Collier-Macmillan Ltd., London (1967).
V. A. Kirillin, V. V. Sychev, and A. E. Sheindlin, Technical Thermodynamics, Energiya, Moscow (1968).
M. W. Chase, “Liquid phase heat capacity,” J. Phys. Chem. Ref. Data, Monograph 9, 1–195 (1998).
E. M. Sparrow, Heat Transfer by Radiation [Russian translation], Energiya, Moscow (1971), p. 295.
A. Yu. Vorobiev, V. A. Petrov, V. E. Titov, and A. P. Chernyshev, “The reflectivity of alumina ceramics with intensive surface heating and subsequent spontaneous cooling,” Teplofiz. Vys. Temp., 45, No. 1, 19–27 (2007).
M. A. Sheindlin and V. N. Senchenko, “Experimental study of the thermodynamic properties of graphite near the melting point,” Dokl. Akad. Nauk SSSR, 298, No. 6, 1383–1386 (1988).
V. P. Skripov, Metastable Liquid, Nauka, Moscow (1972).
V. S. Chirkin, Thermophysical Properties of Nuclear Engineering Materials, Atomizdat, Moscow (1968).
I. V. Sally, Crystallization at Superhigh Cooling Rates, Naukova Dumka, Kiev (1972).
B. I. Kidyarov, Kinetics of the Formation of Crystals from the Liquid Phase, Nauka, Siberian Branch, Novosibirsk (1979).
F. P. Bandy, W. A. Basset, M. S. Weathers, et al., Review article: “The pressure–temperature phase and transformation diagram for carbon; updated through 1994,” Carbon, 34, No. 2, 141–153 (1996).
M. Togaya, “Pressure dependences of the melting temperature of graphite and the electrical resistivity of liquid carbon,” Phys. Rev. Let., 79, No. 13, 2474–2477 (1997).
A. V. Lykov, Theory of Thermal Conductivity, Vysshaya Shkola, Moscow (1967).
P. A. Pavlov, “Calculation of the effective heat of vaporization at explosive boiling up,” Thermophysical Properties of Metastable Systems: Coll. Articles, Ural Scientific Center, USSR Academy of Sciences, Sverdlovsk (1984).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel’naya Tekhnika, No. 6, pp. 48–53, June, 2019.
Rights and permissions
About this article
Cite this article
Kostanovskii, А.V., Kostanovskaya, M.E. Thermophysical Properties of Stable and Supercooled Liquid Carbon. Meas Tech 62, 532–539 (2019). https://doi.org/10.1007/s11018-019-01657-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-019-01657-3