Abstract
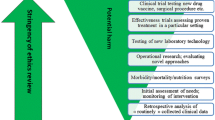
There is an international consensus that medical research involving humans should only be undertaken in accordance with ethical principles. Paradoxically though, there is no consensus over the kinds of activities that constitute research and should be subject to review. In the UK and elsewhere, research requiring review is distinguished from clinical audit. Unfortunately the two activities are not always easy to differentiate from one another. Moreover, as the volume of audit increases and becomes more formal in response to the demand for evidence-based practice in medicine, the overlap between research and audit grows more acute. Arguably, similar ethical standards and systems for ensuring that those standards are met should be applied regardless of whether or not a project is classified as research or audit. At a time when the research ethics review system in the UK is undergoing significant reform it is important that the opportunity is not missed to address the longstanding research-audit problem. We discuss suggestions for further reform that addresses this issue.
Similar content being viewed by others
References
Buxton M.J. “Achievements of Audit in the NHS.” Quality in Health Care 3 (Supplement) (1994): S31–S34. .
Cave, E., and C. Nichols. “Reforming the Ethical Review System: Balancing the Rights and Interests of Research Subjects with the Duty to Facilitate Good Research.” Journal of Clinical Ethics 2(2) 2007: forthcoming.
Central Office for Research Ethics Committees, Governance Arrangements for RECs. London: Department of Health, 2001.
Choo, V. “Thin Line Between Research and Audit.” The Lancet 351 (1998): 337–338: 338.
Clinical Governance Support Team (CGST). A Practical Handbook for Clinical Audit. CGST March 2005.
Committee on Publication Ethics. Code of Conduct for Editors: Checklist and Survey.http://www.publicationethics.org.uk/guidelines/checklist2.doc
Department of Health. A First Class Service: Quality in the New NHS. HSC 1999/033. London: Department of Health, 1999. .
Department of Health. Report of the Ad Hoc Group on the Operation of NHS Research Ethics Committees. London, June 2005.
Godlee F. “Improving On Improvement” BMJ 332 (2006):7549.
Grover S.R., and R. Morley. “Vitamin D deficiency in Veiled or Dark-Skinned Pregnant Women.” Medical Journal of Australia 175(5) (1991): 251–252.
Hayry M., J. Takala, P. Jallinoja, S. Lotjonen, T. Takala, “Ethicalization in Bioscience – A Pilot Study in Finland.” Cambridge Quarterly of Healthcare Ethics 15 (2006): 282–284.
Harris J. “Scientific Research is a Moral Duty.” Journal of Medical Ethics 31 (2005): 242–248.
Hearnshaw H.M., R.M. Harker, F.M. Cheater, R.H. Baker, and G.M. Grimshaw. “Are Audits Wasting Resources by Measuring the Wrong Things? A Survey of Methods Used to Select Audit Review Criteria.” Quality and Safety in Health Care 12 (2003): 24–28.
Hearnshaw H.M. “Comparison of Requirements of Research Ethics Committees in 11 European Countries for a Non-Invasive Interventional Study.” BMJ 328 (2004): 140–141.
Ibrahim J.E. “Translating Quality Into Research: Do we Need More Research into Quality or Should Quality Activities be Conducted Using the Principles and Methodological Rigour of Scientific Research?” Journal of Quality in Clinical Practice 20 (2000): 63–64.
International Committee of Medical Journal Editors. Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication. (1994) at II.F http://www.icmje.org/index.html#privacy
Kopelman L.M. “Minimal Risk as an International Ethical Standard in Research.” Journal of Medicine and Philosophy 29 (2004): 351–378.
Maxwell D.J., and K.I. Kaye. “Multicentre Research: Negotiating the Ethics Approval Obstacle Course.” Medical Journal of Australia 181 (2004): 460.
National Health and Medical Research Council. National Statement on Ethical Conduct in Research Involving Humans. Canberra, 1999.
National Health and Medical Research Council (Australia). When Does Quality Assurance in Health Care Require Independent Ethical Review? Advice to Institutions, Human Research Ethics Committees and Health Care Professionals. Canberra, February 2003.
National Patient Safety Agency and the Central Office for Research Ethics Committees. Differentiating Audit, Service Evaluation and Research. London: Department of Health, 2006.
National Patient Safety Agency and the Central Office for Research Ethics Committees. Building on Improvement: Implementing the Recommendations of the Ad Hoc Advisory Group on the Operation of NHS RECs. London: Department of Health, 2006.
National Health Service. Principles for Best Practice in Clinical Audit, London: Radcliffe Medical Press, 2002.
Rix, G., K. Cutting. “Clinical Audit, The Case For Ethical Scrutiny?” International Journal of Health Care Quality Assurance 9 (1996): 8–20.
Royal College of Physicians of London. Guidelines on the Practice of Ethics Committees in Medical Research Involving Human Subjects. 3rd ed. London: RCP, 1996.
Secretaries of State for Social Services, Wales, Northern Ireland and Scotland. Working for Patients: CM 555. London: HMSO, 1989. .
Secretary of State for Health. Learning from Bristol: The Report of the Public Inquiry into Children's Heart Surgery at the Bristol Royal Infirmary 1984 -1995 Command Paper: CM 5207. London: HMSO, 2001.
Smith R. “Audit and research.” BMJ 315 (1992): 905–906.
Snijders R.J.M., P. Noble, N. Sebire, A. Souka, K.H. Nicolaides, for the Fetal Medicine Foundation First Trimester Screening Group. “UK Multicentre Project on Assessment of Risk Trisomy 21 By Maternal Age and Fetal Nuchal-Translucency Thickness at 10-14 Weeks of Gestation.” The Lancet 352 (1998): 343–46.
Stone D.H. “Proposed Taxonomy of Audit and Related Activities.” Journal of the Royal College of Physicians of London 24 (1990): 30–31. .
United Bristol Healthcare Trust Clinical Audit Central Office. What Is Clinical Audit? Bristol: UBHT, 2005.
Wilson A., G. Grimshaw, R. Baker, J. Thompson. “Differentiating Between Audit and Research: Postal Survey of Health Authorities’ Views.” BMJ 319 (1999): 1235
World Health Organisation. “The Principles of Quality Assurance- Report on Working Group Meeting Convened by Regional Office for Europe (Copenhagen) in Barcelona, 1983.” European Reports and Studies. Copenhagen: WHO, 1985.
United Bristol Healthcare Trust Clinical Audit Central Office. What Is Clinical Audit? v. 2.2. Bristol: UBHT, 2005. .
United Bristol Healthcare Trust Clinical Audit Central Office (UBHT). How to Apply Ethics to Clinical Audit. v 2.2. Bristol: UBHT, 2005.
Acknowledgements
The authors acknowledge the stimulus and support of the European Project (EU-RECA) sponsored by the European Commission, in the preparation of this paper. (DG-Research as part of the Science and Society research programme—6th framework.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cave, E., Nichols, C. Clinical Audit and Reform of the UK Research Ethics Review System. Theor Med Bioeth 28, 181–203 (2007). https://doi.org/10.1007/s11017-007-9034-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11017-007-9034-0