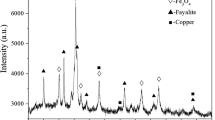
The article presents the results of experimental sulfidization of smelt and converter slags of copper smelting production using granular elemental sulfur at a temperature of 1300 °C in an inert atmosphere without hydrocarbon or metal reducing agents. The distribution of sulfur over products and the behavior of copper are considered. It is shown that feeding elemental sulfur to the slag recovers magnetite and sulfidizes iron, forming a sulfide phase whose yield increases with the consumption of elemental sulfur. An increase in the sulfur consumption leads to a decrease in the concentration of copper in the matte and slag. In the range of sulfur consumption considered, the copper content decreases to less than ~ 0.1% in smelt slag and to ~ 0.2% in converter slag. The concentration of sulfur in the gas is high. The maximum uptake of sulfur by the melt does not exceed ~ 49%. The results obtained indicate the possibility of using elemental sulfur without additional reducing agents to sulfidize copper-smelting slags, either rich or poor in copper, to a high degree of copper recovery.







Similar content being viewed by others
References
C. Doyle, “The steps required to meet production targets at PT Inco, Indonesia: A new innovative business strategy,” in: Proc. TMS Annual Meeting Int. Laterite Nickel Symposium-2004, Charlotte, N. Caroline, USA, March 14–18 (2004), p. 670.
A. Vahed, J. Liu, M. Prokesch, R. Riddle, M. Jafri, R. Barus, and Syukirman, “Testing of nickel laterite smelter dust insufflation. Part 1,” in: Proc Int. Symp. on Pyrometallurgy of Nickel and Cobalt, Sudbery, Canada, August 23–26 (2009), pp. 221–232.
Le Nickel (2010), Nickel Production, www.sln.nc/content/view/75/44/lang.french/Accessed May 19, 2011.
A. V. Vanyukov and N. I. Utkin, Comprehensive Processing of Copper and Nickel Raw Materials [in Russian], Metallurgiya, Chelyabinsk (1988).
A. N. Fedorov, A. A. Komkov, V. N. Bruek, N. A. Gnuskov, and A. P. Kryzhanovskii, “Assimilation of the Vanyukov process for processing oxidized nickel ores at the Southern Urals Nickel Plant,” Tsvetn. Metally, No. 12, 33–37 (2007).
V. V. Shchelkunov, Matte-Forming Processes in Smelting Oxidized Nickel Ores [in Russian], PhD Thesis, 05.16.02, Moscow (2011).
R. A. Pakhomov, Development of Pyrometallurgical Technologies for Processing Oxidized Nickel Ores with Control of the Composition of the Equilibrium Gas Phase [in Russian], PhD Thesis, 05.16.02, Saint Petersburg (2019).
X. Yang, J. Zhang, J. Zhang, et al., “Efficient recovery of copper and cobalt from the matte–slag mixture of ISA furnace by injection of coke and pyrite,” Metall Mater. Trans., B 49, 3118–3126 (2018).
Guo Zhengqi, Pan Jian, Zhu Deqing, and Zhang Feng, “Green and efficient utilization of waste ferric-oxide desulfurizer to clean waste copper slag by the smelting reduction-sulfurizing process,” J. Cleaner Product., 199, 891–899 (2018); ISSN 0959-6526; https://doi.org/10.1016/j.jclepro.2018.07.203.
N. Dosmukhamedov, M. Egizekov, E. Zholdasbay, et al., “Metal recovery from converter slags using a sulfiding agent,” JOM, 70, 2400–2406 (2018); https://doi.org/10.1007/s11837-018-3093-8.
V. I. Karyaev, A. A. Komkov, A. V. Kuznetsov, and I. P. Plotnikov, “Recovering copper and zinc from copper-smelting slags by reduction–sulfidization treatment,” Vest. Magnitogorsk. Gos. Tekhn. Univ. im. G. I. Nosov, 18, No. 2, 4–12 (2020); https://doi.org/10.18503/1995-2732-2020-18-2-4-12.
H.-Y. Wang, G.-H. Zhang, and K.-C. Chou, “Recovery of high-grade copper matte by selective sulfurization of CuO–Fe2O3–SiO2–CaO system,” J. Mater. Res. Technol., https://doi.org/10.1016/j.jmrt.2021.05.085.
N. E. Raimbekov, Reaction of the Components of Sulfides of Copper Raw Materials with Oxygen of Slag Melts in Bath Smelting [in Russian], PhD Thesis, 05.16.03, Moscow (1984).
T. Kh. Khestanov, Phase Equilibriums in Nickel-Containing Sulfide Systems and Reaction of Sulfide Melts with Slag in Bath Smelting [in Russian], PhD Thesis, 05.16.03, Moscow (1985).
D. Shishin, T. Hidayat, A. Fallah-Mehrjardi, et al., “Integrated experimental and thermodynamic modeling study of the effects of Al2O3, CaO, and MgO on slag–matte equilibria in the Cu–Fe–O–S–Si–(Al, Ca, Mg) system,” J. Phase Equilib. Diffus., 40, 445–461 (2019); https://doi.org/10.1007/s11669-019-00716-0.
V. I. Karyaev, A. A. Komkov, A. V. Kuznetsov, I. P. Plotnikov, and V. A. Sokolykh, “Research into the microstructure of industrial copper-containing slags,” Metallurgist, No. 9, 83–92 (2020).
M. Nagamori, “Metal loss to slag: Part I. Sulfidic and oxidic dissolution of copper in fayalite slag from low grade matte,” Metall Mater. Trans., B. 5, 531–538 (1974); https://doi.org/10.1007/BF02644646.
Y. Takeda, “Copper solubility in matte smelting slag,” in: Proc. Int. Conf. Molten Slags, Fluxes Salts 97, 5th, Iron and Steel Soc., Warrendale, PA (1997), pp. 329–339.
A. Yazawa, M. Oida, and Y. Nishikawa, “Distribution equilibria for Ni, Co, As, Sb and Cu between matte and slag,” J. Mining Metallurgical Inst. Japan, 98(1135), 963–968 (1982); https://doi.org/10.2473/shigentosozai1953.98.1135_963.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 67, No. 4, pp. 63–70, April, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Plotnikov, I.P., Komkov, A.A. & Bystrov, S.V. Behavior of Copper and Sulfur During High-Temperature Sulfurization Of Copper-Smelting Slags with Elemental Sulfur. Metallurgist 67, 476–486 (2023). https://doi.org/10.1007/s11015-023-01533-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-023-01533-0