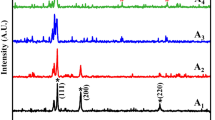
This paper presents the results of experimental and analytical testing of the ternary Ge–In–Sn system. Experimental part includes results of the corrosion resistance, microstructure, hardness and electrical properties of the selected ternary Ge–In–Sn alloys. The prepared alloys were tested using X-ray powder diffraction (XRD) method, scanning electron microscopy with energy dispersive spectrometry (SEMEDS), hardness and electrical conductivity tests. For the obtained values of hardness and electrical conductivity, a mathematical model was used in order to determine the properties of the alloy in the entire range of the composition. The results of the Brinell hardness test show that the Ge80In10Sn10 ternary alloy has the highest hardness of all tested ternary alloys, 254.2 MN/m2. While, results of the electrical conductivity test show that the Ge10In10Sn80 ternary alloy has the highest conductivity of all tested ternary alloys and the highest corrosion resistance. Calculated isothermal section at 25°C, were confirmed with XRD and EDS results.











Similar content being viewed by others
References
S. Raoux, W. Welnic, and D. Lelmini, Chem. Rev., 110, 240–267 (2010).
E. M. Vinod, R. Naik, R. Ganesan, and K. S. Sangunni, J. Non–Cryst. Solids., 358, 2927–2930 (2012).
D. Ielmini and A. L. Lacaita, Mater. Today, 14, 600–607 (2011).
R. Goswami, S. B. Qadri, E. P. Gorzkowski, J. P. Yesinowski, and G. G. Jernigan, Mater Lett., 187, 126–128 (2017).
J. M. del Pozo and L. Díaz, J. Non-Cryst. Solids, 243, 45–51 (1999).
S. L. Ou, C. P. Cheng, C. Y. Yeh, C. J. Chung, K. S. Kao, and R. C. Lin, Adv. Mater. Res., 189–193, 4430–4433 (2011).
P. Moontragoon, N. Vukmirovic, Z. Ikonic, and P. Harrison, J. Appl. Phys., 103, 1037121–1037128 (2008).
V. Ponnambalam, D. T. Morelli, and A Novel, J. Alloys Compd., 740, 42–46 (2018).
P. Nemec, V. Nazabal, A. Moreac, J. Gutwirth, L. Benes, and M. Frumar, Mater. Chem. Phys., 136, 935–941 (2012).
N. Tošković, D. Minić, M. Premović, D. Manasijević, A. Djordjević, and A. Marković, J. Phase Equilib. Diffus., 39, 933–943 (2018).
W. Cao, S. L. Chen, F. Zhang, K. Wu, Y. Yang, Y. A. Chang, R. Schmid–Fetzer, and W. A. Oates, Calphad, 33(2), 328–342 (2009).
Feutelais, B. Legendre, and S. G. Fries, Calphad, 20(1), 109–123 (1996).
P. Y. Chevalier, Thermochim. Acta, 155, 227–240 (1989).
B. J. Lee, C. S. Oh, and J. H. Shim, J. Electron. Mater., 25, 983–991 (1966).
N. Moelans, K. C. Hari Kumar, and P. Wollants, J. Alloys Compd., 360, 98–106 (2003).
V. T. Deshpande and D. B. Sirdeshmukh, Acta Crystallogr., 14, 355–356 (1961).
J. Thewlis and A. R. Davy, Nature, 174, 1011–1011 (1954).
A. S. Cooper, Acta Crystallogr., 15, 578–582 (1962).
S. C. Flower and G. A. Saunders, Philosophical Magazine B, 62, 311–328 (1990).
V. Bhattacharya, K. Chattopadhyay, and J. Nanosci, Nanotechnol., 7, 1736–1742 (2007).
S. F. Bartram, W. G. Moffatt, and B. W. Roberts, J. Less Common Met., 62, 9–12 (1978).
M. J. Anderson and P. J. Whitcomb, RSM: Simplified, Optimizing Processes Using Response Surface Methods for Design of Experiments, Second Edition, CRC Press,Taylot & Francis Group (2017).
G. E. P. Box and N. R. Draper, Response Surfaces, Mixtures, and Ridge Analyses, Second Edition, Wiley (2007).
Stat–Ease, Handbook for Experimenters, Version 11. 00, Stat–Ease, Inc. (2018).
D. C. Montgomery, Design and Analysis of Experiments, Ninth Edition, Wiley (2017).
R. H. Myers, D. C. Mongomery, and C. M. Anderson–Cook, Response Surface Methodology, Process and Product Optimization Using Designed Experiments, Fourth Edition, Wiley (2016).
J. Stanić, Metod Inženjerskih Merenja, Mašinski fakultet, V Prerađeno i dopunjeno izdanje, Beograd (1990).
https://en.wikipedia.org/wiki/Analysis_of_variance, access on 07. 08. 2022.
https://learn.financestrategists.com/financeterms/anova/?gclid=Cj0KCQjwxb2XBhDBARIsAOjDZ37Z_zL85FqJzBP0fojQoZJydvTXZKDRlahJqx5MZlA0b3fDkg-V3oaAl6bEALw_wcBl, access on 07. 08. 2022.
G. E. P. Box and D. R. Cox, “An analysis of transformations,” J. Royal Statistical Society. Series B (Methodological), 26(2), 211–252 (1964).
S. Mallick, Z. Ahmad, F. Touati, J. Bhadra, R. A. Shakoor, and N. J. Al–Thani, “PLA–TiO2 nanocomposites: Thermal, morphological, structural, and humidity sensing properties,” Ceram. Int., 44, 16507–16513 (2018).
D. Q Zhang, L. X. Gao, and G. D. Zhou, “Inhibition of copper corrosion in aerated hydrochloric acid solution by heterocyclic compounds containing a mercapto group,” Corros. Sci., 46, 3031–3040 (2004); https://doi.org/10.1016/j.corsci.2004.04.012.
C. C. Li, X. Y. Guo, S. Shen, P. Song, T. Xu, Y. Wen, and H. F. Yang, “Adsorption and corrosion inhibition of phytic acid calcium on the copper surface in 3 wt% NaCl solution,” Corros. Sci., 83, 147–154 (2014).
A. Popova, S. Raicheva, E. Sokolova, and M. Christov, “Frequency dispersion of the interfacial impedance at mild steel corrosion in acid media in the presence of benzimidazole derivatives,” Langmuir, 12, 2083–2089 (1996); https://doi.org/10.1021/la950148+.
A. Popova, E. Sokolova, S. Raicheva, and M. Christov, “AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives,” Corros. Sci., 45, 33–58 (2003); https://doi.org/10.1016/S0010-938X(02)00072-0.
H. Y. Ma, S. H. Chen, B. S. Yin, S. Y. Zhaom, and X. Q. Liu, Corros. Sci., 45, 867–882 (2003).
S. Deng, X. Li, and X. Xie, “Hydroxymethyl urea and 1,3–bis(hydroxymethyl) urea as corrosion inhibitors for steel in HCl solution,” Corros. Sci., 80, 276–289 (2014), https://doi.org/10.1016/j.corsci.2013.11.041.
C. W. Yan, H. C. Lin, and C. N. Cao, “Investigation of inhibition of 2–mercaptobenzoxazole for copper corrosion,” Electrochim. Acta, 45, 2815–2821 (2000).
R. Zhang, Z. Zhu, X. Leng, J. Pan, and Y. Zhang, “Corrosion characteristic of Cu–10Ni–Fex in 3.5% NaCl,” Int. J. Electrochem. Sci., 13, 11526–11538 (2018).
Acknowledgments
This work has been supported by the National Nature Science Foundation of China (project No. 51950410600) and the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. OI172037). The research presented in this paper was done with the financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia, within the funding of the scientific research work at the University of Belgrade, Technical Faculty in Bor, according to the contract with registration number 451-03-68/2020-14/ 200131.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 66, No. 11, pp. 95–106, November, 2022. Russian https://doi.org/10.52351/00260827_2022_11_95.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zečević, M., Tosković, N., Djordjević, A. et al. Effect of Chemical Composition on the Corrosion Resistance, Microstructure, Hardness and Electrical Conductivity of the Ge–In–Sn Alloys. Metallurgist 66, 1452–1470 (2023). https://doi.org/10.1007/s11015-023-01460-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-023-01460-0