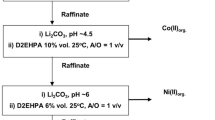
Complex formation of sodium ethylenediaminetetraacetate (EDTA) with cadmium, iron, and nickel, whose presence is possible in starting raw material, is studied. Results obtained make it possible to use EDTA as a complexing solvent in nickel-cadmium battery processing technology.



Similar content being viewed by others
References
E. K. Rudnik and M. P. Nikiel, “Hydrometallurgical recovery of cadmium and nickel from spent Ni–Cd batteries,” Hydrometallurgy, 89, No. 1/2, 61–71 (2007).
A. R. Barashev, S. V. Karelov, O. S. Anisimova, and S. V. Mamyachenkov, “Innovative technology for recycling the negative segments of alkaline batteries using recoverable solvent,” Metallurgist, 55, No. 5/6, 381–385 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 8, pp. 35–36, August, 2015.
Rights and permissions
About this article
Cite this article
Barashev, A.R., Mamyachenkov, S.V., Smirnova, Y.O. et al. Possibility of Using Complexing Reagent for Processing Spent Alkaline Batteries. Metallurgist 59, 664–666 (2015). https://doi.org/10.1007/s11015-015-0156-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-015-0156-8