Abstract
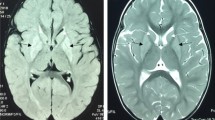
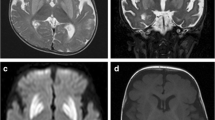
Leigh syndrome (LS) and Leigh-like spectrum are the most common infantile mitochondrial disorders characterized by heterogeneous neurologic and metabolic manifestations. Pathogenic variants in SLC carriers are frequently reported in LS given their important role in transporting various solutes across the blood–brain barrier. SLC19A3 (THTR2) is one of these carriers transporting vitamin-B1 (vitB1, thiamine) into the cell. Targeted NGS of nuclear genes involved in mitochondrial diseases was performed in a patient belonging to a consanguineous Tunisian family with LS and revealed a homozygous c.1264 A > G (p.T422A) variant in SLC19A3. Molecular docking revealed that the p.T422A aa change is located at a key position interacting with vitB1 and causes conformational changes compromising vitB1 import. We further disclosed decreased plasma antioxidant activities of CAT, SOD and GSH enzymes, and a 42% decrease of the mtDNA copy number in patient blood.
Altogether, our results disclose that the c.1264 A > G (p.T422A) variant in SLC19A3 affects vitB1 transport, induces a mtDNA depletion and reduces the expression level of oxidative stress enzymes, altogether contributing to the LS phenotype of the patient.




Similar content being viewed by others
Data Availability
All data are available.
Code Availability
Not applicable.
References
Aebi H (1984) Catalase in vitro. Methods in enzymology, vol 105. Academic press, pp 121–126
Alfadhel M, Almuntashri M, Jadah R, Bashiri F, Al Rifai M, Al Shalaan H, Al Balwi M, Al Rumayan A, Eyaid W, Al-Twaijri W (2013) Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J Rare Dis 8:1–8
Alfadhel M, Umair M, Almuzzaini B, Alsaif S, AlMohaimeed S, Almashary M, Alharbi W, Alayyar L, Alasiri A, Ballow M, Al Abdulrahman A, Alaujan M, Nashabat M, Al-Odaib A, Altwaijri W, Al-Rumayyan A, Alrifai M, Alfares A, AlBalwi M, Tabarki B (2019) Targeted SLC19A3 gene sequencing of 3000 saudi newborn: a pilot study toward newborn screening. Ann Clin Transl Neurol 6(10):2097–2103
Asada K, Takahashi MA, Nagate M (1974) Assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 38(2):471–473
Baertling F, Rodenburg R, Schaper J, Smeitink J, Koopman W, Mayatepek E, Morava E, Distelmaier F (2014) A guide to diagnosis and treatment of Leigh syndrome. J Neurol Neurosurg Psychiatry 85(3):257–265
Bakare AB, Lesnefsky EJ, Iyer S (2021) Leigh syndrome: a tale of two genomes. Front Physiol 12:693734
Biovia, D. S (2016) Discovery studio. Dassault Systémes BIOVIA.
Butterworth RF (1989) Effects of thiamine deficiency on brain metabolism: implications for the pathogenesis of the Wernicke-Korsakoff syndrome. Alcohol Alcohol 24(4):271–279
Chang X, Wu Y, Zhou J, Meng H, Zhang W, Guo J (2020) A meta-analysis and systematic review of Leigh syndrome: clinical manifestations, respiratory chain enzyme complex deficiency, and gene mutations. Medicine, 99(5)
Croft L, Napoli E, Hung CK, Gearhart S, Heym K, Wong S et al (2013) Clinical and biochemical characterization of thiamine deficiency in Pacific harbor seals (Phoca vitulina) maintained at a zoological facility. J Am Vet Med Assoc 243:1179–1189
De Freitas-Silva DM, de Souza Resende L, Pereira SRC, Franco GC, Ribeiro AM (2010) Maternal thiamine restriction during lactation induces cognitive impairments and changes in glutamate and GABA concentrations in brain of rat offspring. Behav Brain Res 211(1):33–40
Devivo DC, Haymond MW, Obert KA, Nelson JS, Pagliara AS (1979) Defective activation of the pyruvate dehydrogenase complex in subacute necrotizing encephalomyelopathy (Leigh disease). Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 6(6):483–494
Dhir S, Tarasenko M, Napoli E, Giulivi C (2019) Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front Psychiatry 10:207
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods in enzymology, vol 186. Academic press, pp 421–431
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Evans OB (1981) Pyruvate decarboxylase deficiency in subacute necrotizing encephalomyelopathy. Arch Neurol 38(8):515–519
Felhi R, Charif M, Sfaihi L, Mkaouar-Rebai E, Desquiret-Dumas V, Kallel R, …, Lenaers G (2020) Mutations in aARS genes revealed by targeted next-generation sequencing in patients with mitochondrial diseases. Mol Biol Rep 47:3779–3787
Gerards M, Kamps R, van Oevelen J, Boesten I, Jongen E, de Koning B, …, Smeets H (2013) Exome sequencing reveals a novel moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain 136(3):882–890
Gerards M, Sallevelt SC, Smeets HJ (2016) Leigh syndrome: resolving the clinical and genetic heterogeneity paves the way for treatment options. Mol Genet Metab 117(3):300–312
Goudenège D, Bris C, Hoffmann V, Desquiret-Dumas V, Jardel C, Rucheton B, Bannwarth S, Paquis-Flucklinger V, Lebre A, Colin E, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Procaccio V (2019) eKLIPse: a sensitive tool for the detection and quantification of mitochondrial DNA deletions from next-generation sequencing data. Genet Sci 21(6):1407–1416
Gschwend DA, Good AC, Kuntz ID (1996) Molecular docking towards drug discovery. J Mol Recognition: Interdisciplinary J 9(2):175–186
Hechmi M, Charif M, Kraoua I, Fassatoui M, Dallali H, Desquiret-Dumas V, Bris C, Goudenège D, Drissi C, Galaï S, Ouerhani S, Procaccio V, Amati-Bonneau P, Abdelhak S, Youssef-Turki B, Lenaers I G, Kefi R (2022) Next-generation sequencing of tunisian Leigh syndrome patients reveals novel variations: impact for diagnosis and treatment. Biosci Rep 42(9):BSR20220194
Lake NJ, Compton AG, Rahman S, Thorburn DR (2016) Leigh syndrome: one disorder, more than 75 monogenic causes. Ann Neurol 79(2):190–203
Lee A, Kim D (2020) CRDS: consensus reverse docking system for target fishing. Bioinformatics 36(3):959–960
Leigh D (1951) Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry 14(3):216
Lewin HA, Biotechniques (1992) 13 522–524
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maas RR, Iwanicka-Pronicka K, Kalkan Ucar S, Alhaddad B, AlSayed M, Al‐Owain MA, Wortmann SB (2017) Progressive deafness–dystonia due to SERAC1 mutations: a study of 67 cases. Ann Neurol 82(6):1004–1015
Nga, N. T. T., & Quang, D. D (2019) Unraveling the antioxidant potential of thiamine: Thermochemical and kinetics studies in aqueous phase using DFT. Vietnam Journal of Chemistry, 57(4): 485–490.
Ogawa, E., Fushimi, T., Ogawa‐Tominaga, M., Shimura, M., Tajika, M., Ichimoto, K., ... & Murayama, K (2020) Mortality of Japanese patients with Leigh syndrome: effects of age at onset and genetic diagnosis. Journal of Inherited Metabolic Disease, 43(4): 819–826.
Ortigoza-Escobar JD, Molero-Luis M, Arias A, Oyarzabal A, Darín N, Serrano M, Garcia-Cazorla A, Tondo M, Hernandez M, Garcia-Villoria J, Gort C, Mayr L, Rodríguez-Pombo JA, Ribes P, Artuch A R, Perez-Duenas B (2016) Free-thiamine is a potential biomarker of thiamine transporter-2 deficiency: a treatable cause of Leigh syndrome. Brain 139(1):31–38
Ortigoza-Escobar JD, Serrano M, Molero M, Oyarzabal A, Rebollo M, Muchart J, Artuch R, Rodríguez-Pombo P, Pérez-Dueñas B (2014) Thiamine transporter-2 deficiency: outcome and treatment monitoring. Orphanet J Rare Dis 9:1–10
Ozand PT, Gascon GG, Essa A, Joshi M, Al Jishi S, Bakheet E, Al Watban S, Kawi J M, Dabbagh O (1998) Biotin-responsive basal ganglia disease: a novel entity. Brain 121(7):1267–1279
Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P (2008) Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J 49(8):640
Ruhoy IS, Saneto RP (2014) The genetics of Leigh syndrome and its implications for clinical practice and risk management. The Application of Clinical Genetics, 221–234
Smith TJ, Johnson CR, Koshy R, Hess SY, Qureshi UA, Mynak ML, Fischer PR (2021) Thiamine deficiency disorders: a clinical perspective. Ann N Y Acad Sci 1498(1):9–28
Stendel C, Neuhofer C, Floride E, Yuqing S, Ganetzky RD, Park J, Freisinger P, Kornblum C, Schöls L, Distelmaier F, Stettner G, Büchner B, Falk M, Mayr A, Synofzik M, Abicht A, Haack B, Prokisch H, Wortmann B, Murayama K, Fang F, Thomas K (2020) Delineating MT-ATP6-associated disease: from isolated neuropathy to early onset neurodegeneration. Neurol Genet, 6(1)
Todd KG, Butterworth RF (1999) Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia 25(2):190–198
Vernau K, Napoli E, Wong S, Ross-Inta C, Cameron J, Bannasch D, Bollen A, Dickinson P, Giulivi C (2015) Thiamine Deficiency‐Mediated brain mitochondrial Pathology in alaskan huskies with mutation in SLC19A3. 1. Brain Pathol 25(4):441–453
Wang, J., Wang, J., Han, X., Liu, Z., Ma, Y., Chen, G., ... & Fang, F (2021) Report of the largest Chinese cohort with SLC19A3 gene defect and literature review. Frontiers in genetics, 12, 683255.
Worsley HE, Brookfield RW, Elwood JS, Noble RL, Taylor WH (1965) Lactic acidosis with necrotizing encephalopathy in two sibs. Arch Dis Child 40(213):492
Zeng WQ, Al-Yamani E, Acierno JS, Slaugenhaupt S, Gillis T, MacDonald ME, Ozand PT, Gusella JF (2005) Biotin-responsive basal ganglia disease maps to 2q36. 3 and is due to mutations in SLC19A3. Am J Hum Genet 77(1):16–26
Acknowledgements
We would like to thank all the member of the family for their cooperation in the present study. This work was supported by the Ministry of Higher Education and Scientific Research in Tunisia, University of Sfax. We acknowledge the support from the INSERM and CNRS, France, the Universty of Angers, the University Hospital of Angers, the Pays of Loire Region, France.
Funding
This work was supported by the Ministry of Higher Education and the Scientific Research in Tunisia.
Author information
Authors and Affiliations
Contributions
Felhi R, Charif M, Lenaers G and Fakhfakh F had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Sfaihi L, Kamoun TH: Clinical investigations. Fakher F: molecular docking. Aoiadni N: oxidative stress. Analysis or interpretation of data: Felhi R, and Fakhfakh F. Draft of the manuscript: Felhi F, Charif M, Lenaers L and Fakhfakh F.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest or financial relationships relevant to this article to disclose. The authors alone are responsible for the content and writing of the paper. No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethics approval
The study was conducted in accordance with the principles stated in the Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects, Helsinki, Finland, 1964, and as amended in Fortaleza, Brazil, 2013. The study design was approved by the committee on research ethics of the University of Sfax, Tunisia.
Consent to participate
Informed consent for publication of this study was obtained from all patients and/or families involved.
Consent for publication
We certifie that all contributors have read and approved the submission to this journal and that there is no financial or commercial involvement, or other conflict of interest by any author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Felhi, R., Sfaihi, L., Charif, M. et al. Vitamin B1 deficiency leads to high oxidative stress and mtDNA depletion caused by SLC19A3 mutation in consanguineous family with Leigh syndrome. Metab Brain Dis 38, 2489–2497 (2023). https://doi.org/10.1007/s11011-023-01280-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01280-w