Abstract
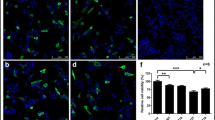
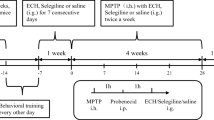
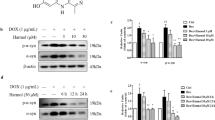
Alpha-Synuclein (α-Syn) accumulation is central to the pathogenesis of Parkinson’s disease (PD), hence the quest for finding potential therapeutics that may promote the α-Syn clearance is the need of the hour. To this, activation of the evolutionarily conserved protein and key regulator of the autophagy, 5’AMP-activated protein kinase (AMPK) is well-known to induce autophagy and subsequently the clearance of α-Syn aggregates. Alpha-mangostin (AM) a polyphenolic xanthone obtained from Garcinia Mangostana L. was previously reported to activate AMPK-dependent autophagy in various pre-clinical cancer models. However, no studies evidenced the effect of AM on AMPK-dependent autophagy activation in the PD. Therefore, the present study aimed to investigate the neuroprotective activity of AM in the chronic rotenone mouse model of PD against rotenone-induced α-Syn accumulation and to dissect molecular mechanisms underlying the observed neuroprotection. The findings showed that AM exerts neuroprotection against rotenone-induced α-Syn accumulation in the striatum and cortex by activating AMPK, upregulating autophagy (LC3II/I, Beclin-1), and lysosomal (TFEB) markers. Of note, an in-vitro study utilizing rat pheochromocytoma cells verified that AM conferred the neuroprotection only through AMPK activation, as the presence of inhibitors of AMPK (dorsomorphin) and autophagy (3-methyl adenine) failed to mitigate rotenone-induced α-Syn accumulation. Moreover, AM also counteracted rotenone-induced behavioral deficits, oxidative stress, and degeneration of nigro-striatal dopaminergic neurons. In conclusion, AM provided neuroprotection by ameliorating the rotenone-induced α-Syn accumulation through AMPK-dependent autophagy activation and it can be considered as a therapeutic agent which might be having a higher translational value in the treatment of PD.









Similar content being viewed by others
Data availability
The data that supports the findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
- AM:
-
Alpha-mangostin
- AMPK:
-
5’AMP-activated protein kinase
- ANOVA:
-
Analysis of variance
- APS:
-
Ammonium persulfate
- α-Syn:
-
Alpha-Synuclein
- Atg:
-
Autophagy-related gene
- BCA:
-
Bicinchoninic acid
- CMC:
-
Carboxymethylcellulose
- CPCSEA:
-
Committee for the Purpose of Control and Supervision of Experiments on Animals
- DAergic:
-
Dopaminergic
- DTNB:
-
5,5-dithiobis (2-nitrobenzoic acid)
- GSH:
-
Reduced glutathione
- H2O2 :
-
Hydrogen peroxide
- HRP:
-
Horseradish peroxidase
- IAEC:
-
Institutional Animal Ethics Committee
- LBs:
-
Lewy bodies
- LC3B:
-
Light chain-3B
- 3-MA:
-
3-methyl adenine
- MDA:
-
Malondialdehyde
- MPP+ :
-
1-methyl-4-phenylpyridinium
- OD:
-
Optical density
- PBS:
-
Phosphate buffer saline
- PC12:
-
Pheochromocytoma
- PD:
-
Parkinson’s disease
- PEG:
-
Polyethylene glycol
- PFA:
-
Paraformaldehyde
- PMSF:
-
Phenylmethyl sulfonyl fluoride
- PVDF:
-
Polyvinylidene difluoride
- RBW:
-
Round beam walk
- RIPA:
-
Radioimmunoprecipitation assay
- ROS:
-
Reactive oxygen species
- SDS:
-
Sodium dodecyl sulfate
- SH-SY5Y:
-
Neuroblastoma
- SLS:
-
Sodium lauryl sulfate
- SNc:
-
Substantia nigra pars compacta
- TBA:
-
Thiobarbituric acid
- TBARS:
-
TBA-reactive substance assay
- TEMED:
-
Tetramethyl-ethylenediamine
- TFEB:
-
Transcription factor-EB
- TH:
-
Tyrosine hydroxylase
- Ulk-1:
-
Unc-51 like autophagy activating kinase
- VTA:
-
Ventral tegmental area
References
Abdulwahid Arif I, Ahmad Khan H (2010) Environmental toxins and Parkinson’s disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health 26:121–128. https://doi.org/10.1177/0748233710362382
Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM et al (2010) Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol 67:1464–1472. https://doi.org/10.1001/ARCHNEUROL.2010.198
Aman Y, Schmauck-Medina T, Hansen M et al (2021) Autophagy in healthy aging and disease. Nat Aging 2021 1(8 1):634–650. https://doi.org/10.1038/s43587-021-00098-4
Arawaka S, Sato H, Sasaki A et al (2017) Mechanisms underlying extensive Ser129-phosphorylation in α-synuclein aggregates. Acta Neuropathol Commun 5:48. https://doi.org/10.1186/S40478-017-0452-6/FIGURES/9
Arotcarena M-L, Teil M, Dehay B (2019) Autophagy in synucleinopathy: the overwhelmed and defective machinery. Cells 8:565. https://doi.org/10.3390/CELLS8060565
Bartels T, Choi JG, Selkoe DJ (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477:107–111. https://doi.org/10.1038/NATURE10324
Chao AC, Hsu YL, Liu CK, Kuo PL (2011) α-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J Agric Food Chem 59:2086–2096. https://doi.org/10.1021/JF1042757
Chen P, Wang Y, Chen L et al (2020) Apelin-13 protects dopaminergic neurons against rotenone—Induced neurotoxicity through the AMPK/mTOR/ULK-1 mediated autophagy activation. Int J Mol Sci 21:8376. https://doi.org/10.3390/IJMS21218376
Curry DW, Stutz B, Andrews ZB, Elsworth JD (2018) Targeting AMPK Signaling as a Neuroprotective Strategy in Parkinson’s disease. J Parkinsons Dis 8:161–181. https://doi.org/10.3233/JPD-171296
Decressac M, Mattsson B, Weikop P et al (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci U S A. https://doi.org/10.1073/PNAS.1305623110/-/DCSUPPLEMENTAL/PNAS.201305623SI.PDF
Dehay B, Bové J, Rodríguez-Muela N et al (2010) Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 30:12535–12544. https://doi.org/10.1523/JNEUROSCI.1920-10.2010
Dexter DT, Sian J, Rose S et al (1994) Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol 35:38–44. https://doi.org/10.1002/ANA.410350107
Dulovic M, Jovanovic M, Xilouri M et al (2014) The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol Dis 63:1–11. https://doi.org/10.1016/J.NBD.2013.11.002
El-Ghaiesh SH, Bahr HI, Ibrahiem AT et al (2020) Metformin protects from rotenone–Induced nigrostriatal neuronal death in adult mice by activating AMPK-FOXO3 signaling and mitigation of angiogenesis. Front Mol Neurosci 13:84. https://doi.org/10.3389/FNMOL.2020.00084/BIBTEX
Estrada-Valencia R, Hurtado-Díaz ME, Rangel-López E et al (2022) Alpha-mangostin alleviates the short-term 6-hydroxydopamine-induced neurotoxicity and oxidative damage in rat cortical slices and in Caenorhabditis elegans. Neurotox Res 40:573–584. https://doi.org/10.1007/S12640-022-00493-8
Foffani G, Obeso JA (2018) A cortical pathogenic theory of Parkinson’s disease. Neuron 99:1116–1128. https://doi.org/10.1016/J.NEURON.2018.07.028
Gao J, Perera G, Bhadbhade M et al (2019) Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells. J Biol Chem 294:14241–14256. https://doi.org/10.1074/JBC.RA119.008733
Gao Z, Wang H, Zhang B et al (2018) Trehalose inhibits H2O2-induced autophagic death in dopaminergic SH-SY5Y cells via mitigation of ROS-dependent endoplasmic reticulum stress and AMPK activation. Int J Med Sci 15:1014–1024. https://doi.org/10.7150/ijms.25656
Ghanem SS, Fayed HS, Zhu Q et al (2021) Natural alkaloid compounds as inhibitors for alpha-synuclein seeded fibril formation and toxicity. Molecules 26:3736. https://doi.org/10.3390/MOLECULES26123736
Goedert M, Jakes R, Spillantini MG (2017) The synucleinopathies: twenty years on. J Parkinson’s Disease 7:S51. https://doi.org/10.3233/JPD-179005
Guo YL, Duan WJ, Lu DH et al (2021) Autophagy-dependent removal of α-synuclein: a novel mechanism of GM1 ganglioside neuroprotection against Parkinson’s disease. Acta Pharmacol Sin 42:518–528. https://doi.org/10.1038/S41401-020-0454-Y
Hang L, Wang Z, Foo ASC et al (2021) Conditional disruption of AMP kinase in dopaminergic neurons promotes Parkinson’s disease-associated phenotypes in vivo. Neurobiol Dis 161:105560. https://doi.org/10.1016/J.NBD.2021.105560
Hao XM, da Li L, Duan CL, Li YJ (2017) Neuroprotective effect of α-mangostin on mitochondrial dysfunction and α-synuclein aggregation in rotenone-induced model of Parkinson’s disease in differentiated SH-SY5Y cells. J Asian Nat Prod Res 19:833–845. https://doi.org/10.1080/10286020.2017.1339349
Herrera-Aco DR, Medina-Campos ON, Pedraza-Chaverri J et al (2019) Alpha-mangostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem Toxicol 124:300–315. https://doi.org/10.1016/J.FCT.2018.12.018
Huang J, Wang X, Zhu Y et al (2019) Exercise activates lysosomal function in the brain through AMPK-SIRT1‐TFEB pathway. CNS Neurosci Ther 25:796. https://doi.org/10.1111/CNS.13114
Ibrahim MY, Hashim NM, Mariod AA et al (2016) α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab J Chem 9:317–329. https://doi.org/10.1016/J.ARABJC.2014.02.011
Jang M, Park R, Kim H et al (2018) AMPK contributes to autophagosome maturation and lysosomal fusion. Sci Rep 8. https://doi.org/10.1038/S41598-018-30977-7
Janhom P, Dharmasaroja P (2015) Neuroprotective effects of alpha-mangostin on MPP+-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J Toxicol 2015. https://doi.org/10.1155/2015/919058
Jewett M, Jimenez-Ferrer I, Swanberg M (2017) Astrocytic expression of GSTA4 is associated to dopaminergic neuroprotection in a rat 6-OHDA Model of Parkinson’s disease. Brain Sci 7. https://doi.org/10.3390/BRAINSCI7070073
Khairnar A, Plumitallo A, Frau L et al (2010) Caffeine enhances astroglia and microglia reactivity induced by 3,4-methylenedioxymethamphetamine (‘ecstasy’) in mouse brain. Neurotox Res 17:435–439. https://doi.org/10.1007/S12640-009-9125-Y
Kühn K, Wellen J, Link N et al (2003) The mouse MPTP model: gene expression changes in dopaminergic neurons. Eur J Neurosci 17:1–12. https://doi.org/10.1046/J.1460-9568.2003.02408.X
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38. https://doi.org/10.1038/NRN3406
Lu M, Su C, Qiao C et al (2016) Metformin prevents dopaminergic neuron death in MPTP/P-induced mouse model of Parkinson’s disease via autophagy and mitochondrial ROS clearance. Int J Neuropsychopharmacol 19:1–11. https://doi.org/10.1093/ijnp/pyw047
Mahul-Mellier AL, Burtscher J, Maharjan N et al (2020) The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A 117:4971–4982. https://doi.org/10.1073/PNAS.1913904117/-/DCSUPPLEMENTAL
Muntané G, Dalfó E, Martinez A, Ferrer I (2008) Phosphorylation of tau and alpha-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related alpha-synucleinopathies. Neuroscience 152:913–923. https://doi.org/10.1016/J.NEUROSCIENCE.2008.01.030
Murphy KE, Gysbers AM, Abbott SK et al (2015) Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov Disord 30:1639–1647. https://doi.org/10.1002/MDS.26141
Pan-Montojo F, Anichtchik O, Dening Y et al (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5:e8762. https://doi.org/10.1371/JOURNAL.PONE.0008762
Pan-Montojo F, Schwarz M, Winkler C et al (2012) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. https://doi.org/10.1038/srep00898
Parekh P, Sharma N, Gadepalli A et al (2019) A cleaning crew: the pursuit of autophagy in Parkinson’s disease. ACS Chem Neurosci 10:3914–3926. https://doi.org/10.1021/ACSCHEMNEURO.9B00244
Parkhe A, Parekh P, Nalla LV et al (2020) Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson’s disease. Neurosci Lett 716. https://doi.org/10.1016/J.NEULET.2019.134652
Paxinos G, Franklin KBJ (2008) The Mouse Brain in Stereotaxic Coordinates, 3rd edn. Academic Press, San Diego, CA
Phillipson OT (2017) Alpha-synuclein, epigenetics, mitochondria, metabolism, calcium traffic, & circadian dysfunction in Parkinson’s disease. An integrated strategy for management. Ageing Res Rev 40:149–167. https://doi.org/10.1016/J.ARR.2017.09.006
Pingale T, Gupta GL (2020) Classic and evolving animal models in Parkinson’s disease. Pharmacol Biochem Behav 199:173060. https://doi.org/10.1016/J.PBB.2020.173060
Poewe W, Seppi K, Tanner CM et al (2017) Parkinson disease. Nat Rev Dis Primers 3:1–21. https://doi.org/10.1038/NRDP.2017.13
Ramirez-Moreno MJ, Duarte-Jurado AP, Gopar-Cuevas Y et al (2019) Autophagy stimulation decreases dopaminergic neuronal death mediated by oxidative stress. Mol Neurobiol 56:8136–8156. https://doi.org/10.1007/S12035-019-01654-1
Reznick RM, Zong H, Li J et al (2007) Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5:151–156. https://doi.org/10.1016/J.CMET.2007.01.008
Sakamula R, Yata T, Thong-asa W (2022) Nanostructure lipid carriers enhance alpha-mangostin neuroprotective efficacy in mice with rotenone-induced neurodegeneration. Metab Brain Dis. https://doi.org/10.1007/S11011-022-00967-W
Sala G, Arosio A, Stefanoni G et al (2013) Rotenone upregulates alpha-synuclein and myocyte enhancer factor 2D independently from lysosomal degradation inhibition. BioMed Res Int. https://doi.org/10.1155/2013/846725
Sharma M, Kaur J, Rakshe S et al (2022) Intranasal exposure to low-dose rotenone induced alpha-synuclein accumulation and Parkinson’s like symptoms without loss of dopaminergic neurons. Neurotox Res 40:215–229. https://doi.org/10.1007/S12640-021-00436-9/FIGURES/7
Spencer B, Potkar R, Trejo M et al (2009) Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci 29:13578–13588. https://doi.org/10.1523/JNEUROSCI.4390-09.2009
Tenreiro S, Reimão-Pinto MM, Antas P et al (2014) Phosphorylation modulates clearance of alpha-synuclein inclusions in a yeast model of Parkinson’s disease. PLoS Genet 10:e1004302. https://doi.org/10.1371/JOURNAL.PGEN.1004302
Tieu K (2011) A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. https://doi.org/10.1101/CSHPERSPECT.A009316
Wang F, Ma H, Liu Z et al (2017) α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed pharmacotherapy = Biomedecine pharmacotherapie 92:672–680. https://doi.org/10.1016/J.BIOPHA.2017.05.129
Wang SJ, Wang Q, Ma J et al (2018) Effect of moxibustion on mTOR-mediated autophagy in rotenone-induced Parkinson’s disease model rats. Neural Regen Res 13:112–118. https://doi.org/10.4103/1673-5374.224380
Wang Y, Liu J, Chen M et al (2016) The novel mechanism of rotenone-induced α-synuclein phosphorylation via reduced protein phosphatase 2A activity. Int J Biochem Cell Biol 75:34–44. https://doi.org/10.1016/J.BIOCEL.2016.03.007
Wood SJ, Wypych J, Steavenson S et al (1999) alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem 274:19509–19512. https://doi.org/10.1074/JBC.274.28.19509
Wu Y, Li X, Zhu JX et al (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 19:163–174. https://doi.org/10.1159/000328516
Zhao DY, Yu DD, Ren L, Bi GR (2020) Ligustilide protects PC12 cells from oxygen-glucose deprivation/reoxygenation-induced apoptosis via the LKB1-AMPK-mTOR signaling pathway. Neural Regen Res 15:473–481. https://doi.org/10.4103/1673-5374.266059
Zhou Q, Chen B, Wang X et al (2016) Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep 6. https://doi.org/10.1038/SREP32206
Funding
This work was supported by the seed fund of the National Institute of Pharmaceutical Education and Research (NIPER), Ahmedabad. Dr. Amit Khairnar gratefully acknowledges the support of the Ramalingaswami Fellowship from the Department of Biotechnology, India.
Author information
Authors and Affiliations
Contributions
Both P.P. and N.S. contributed equally to this work. We declare that all authors made fundamental contributions to the manuscript. P.P. and A.M.K. designed the study. P.P., N.S., A.G., M.S., and A.A.S. conducted experiments. P.P., N.S., and A.G. analyzed the data. P.P., N.S., M.S., S.C., and A.M.K. participated in the interpretation and writing of the manuscript. A.K. and A.M.K. advised the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
IAEC approval number: IAEC/2018/033.
Consent to participate
Not applicable.
Consent for publication
Each author has provided his/her consent for this publication.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
Additional results of behavioral assessment. (PDF 389 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parekh, P., Sharma, N., Sharma, M. et al. AMPK-dependent autophagy activation and alpha-Synuclein clearance: a putative mechanism behind alpha-mangostin’s neuroprotection in a rotenone-induced mouse model of Parkinson’s disease. Metab Brain Dis 37, 2853–2870 (2022). https://doi.org/10.1007/s11011-022-01087-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01087-1