Abstract
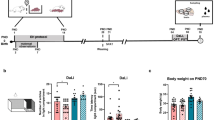
The perinatal depression exposes the child to antidepressants during vulnerable window of development, which can chronically impact the mental wellbeing of new born. Active pharmaceuticals are not tested for this long term neurobehavioral aspect of toxicity during drug development process. Keeping this in view, the current study was designed to study the effect of pre-weaning fluoxetine exposure on depression-like behavior of the offspring upon attaining adulthood using FST (Forced swim test). Additionally, the brain tryptophan, 5-HT (5-hydroxytryptamine) and its metabolite 5-HIAA (5-hydroxyindoleacetic acid) levels were quantified using Enzyme linked Immunosorbent Assay (ELISA), while expression of SERT (serotonin receptor), 5-HT1A receptor, TPH (tryptophan hydroxylase) genes were monitored using qPCR. Our data showed that pre-weaning fluoxetine (10, 50 or 100 mg/kg) exposure decreased depression-like behavior. The 5-HT and 5-HIAA levels showed declining trend. However, the 5-HT synthetic precursor i.e. tryptophan levels were found to be significantly elevated in both brain and plasma as compared to control rats. The gene expression study did not reveal any significant alterations as compared to control. In conclusion, the present study demonstrate that pre-weaning fluoxetine exposure decreased depression-like behavior upon adulthood via perturbing tryptophan metabolism.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to fact that the study is the part of ongoing research but are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Abbas G, Naqvi S, Mehmood S, Kabir N, Dar A (2011) Forced swimming stress does not affect monoamine levels and neurodegeneration in rats. Neurosci Bull 27(5):319–324
Abbas G, Naqvi S, Dar A (2012) Comparison of monoamine reuptake inhibitors for the immobility time and serotonin levels in the hippocampus and plasma of sub-chronically forced swim stressed rats. Pak J Pharm Sci 25:441–445
Abbott LC, Nigussie F (2020) Adult neurogenesis in the mammalian dentate gyrus. Anat Histol Embryol 49(1):3–16
Albert PR, Vahid-Ansari F, Luckhart C (2014) Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre-and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 8:199
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27(1–2):3–18
Atif M, Halaki M, Raynes-Greenow C, Chow CM (2021) Perinatal depression in Pakistan: A systematic review and meta-analysis. Birth 48(2):149–163
Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V (2006) Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology 31(4):814–824
Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA (2006) Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354(6):579–587
Cipriani A, Brambilla P, Furukawa TA, Geddes J, Gregis M, Hotopf M, Malvini L, Barbui C (2005) Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev (4):CD004185
Cooper WO, Willy ME, Pont SJ, Ray WA (2007) Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 196(6):544. e541-544. e545
de Oliveira CL, Bolzan JA, Surget A, Belzung C (2020) Do antidepressants promote neurogenesis in adult hippocampus? A systematic review and meta-analysis on naive rodents. Pharmacol Ther 210:107515
Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC (2005) Perinatal depression: prevalence, screening accuracy, and screening outcomes: summary. AHRQ Evid Rep Summ 119:1–35
Gronemann FH, Petersen J, Alulis S, Jensen KJ, Riise J, Ankarfeldt MZ, Solem EJ, Bødker N, Osler M (2021) Treatment patterns in patients with treatment-resistant depression in Danish patients with major depressive disorder. J Affect Disord 287:204–213
Hemels ME, Einarson A, Koren G, Lanctôt KL, Einarson TR (2005) Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta-analysis. Ann Pharmacother 39(5):803–809
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858
Kendall K, Van Assche E, Andlauer T, Choi K, Luykx J, Schulte E, Lu Y (2021) The genetic basis of major depression. Psychol Med 51(13):2217–2230
Klaassen CD, Watkins JB (2010) Casarett & Doull's essentials of toxicology, 3rd Edition, McGraw-Hill Medical Pub. Division
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23(10):1289–1291
Levone BR, Moloney GM, Cryan JF, O’Leary OF (2021) Specific sub-regions along the longitudinal axis of the hippocampus mediate antidepressant-like behavioral effects. Neurobiol Stress 14:100331
Licht T, Kreisel T, Biala Y, Mohan S, Yaari Y, Anisimov A, Alitalo K, Keshet E (2020) Age-dependent remarkable regenerative potential of the dentate gyrus provided by intrinsic stem cells. J Neurosci 40(5):974–995
Macedo NJ, Ferreira TL (2014) Maximizing total RNA yield from TRIzol reagent protocol: a feasibility study. ASEE Zone I Conference
Mann JJ, McBride PA, Anderson GM, Mieczkowski TA (1992) Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and life-time psychopathology. Biol Psychiat 32(3):243–257
Millard SJ, Lum JS, Fernandez F, Weston-Green K, Newell KA (2019) Perinatal exposure to fluoxetine increases anxiety-and depressive-like behaviours and alters glutamatergic markers in the prefrontal cortex and hippocampus of male adolescent rats: A comparison between Sprague-Dawley rats and the Wistar-Kyoto rat model of depression. J Psychopharmacol 33(2):230–243
Mirowsky J (1996) Age and the gender gap in depression. J Health Soc Behav 37:362–380
Mirowsky J, Ross CE (1992) Age and depression. J Health Soc Behav 33(3):187–205
Nadeem, Siddiqui RA, Usman S, Nisar U, Abbas G (2021) Pre-weaning fluoxetine exposure perturbs social behavior at adulthood via altering hippocampal morphometry and PSD-95 expression in rats. Pak J Pharm Sci 34:795–802
Nikolac Perkovic M, Sagud M, Tudor L, Konjevod M, Svob Strac D, Pivac N (2021) A load to find clinically useful biomarkers for depression. Major depressive disorder, Springer 1305:175–202
Owens MJ (2004) Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry 65:5–10
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266(5604):730–732
Ramsteijn AS, Jašarević E, Houwing DJ, Bale TL, Olivier JD (2020) Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes 11(4):735–753
Rosa-Neto P, Diksic M, Okazawa H, Leyton M, Ghadirian N, Mzengeza S, Nakai A, Debonnel G, Blier P, Benkelfat C (2004) Measurement of brain regional α-[11C] Methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-freepatients with major depression. Arch Gen Psychiatry 61(6):556–563
Rossi A, Barraco A, Donda P (2004) Fluoxetine: a review on evidence based medicine. Ann Gen Hosp Psychiatry 3(1):1–8
Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M (2021) Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci 22(6):2973
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C T method. Nat Protoc 3(6):1101
Shi M, Sun H, Xu Y, Wang Z, Cui H, Wang C, Liu W, An G, Hu J (2017) Methylation status of the serotonin transporter promoter CpG island is associated with major depressive disorder in Chinese Han population: a case-control study. J Nerv Ment Dis 205(8):641–646
Ulbrich B, Palmer AK (1996) Neurobehavioral aspects of developmental toxicity testing. Environ Health Perspect 104(suppl 2):407–412
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40(15):e115–e115
WHO (2012) Risks to mental health: An overview of vulnerabilities and risk factors. Geneva, Switzerland pp 1–14
Więdłocha M, Marcinowicz P, Janoska-Jaździk M, Szulc A (2021) Gut microbiota, kynurenine pathway and mental disorders–Review. Prog Neuro-Psychopharmacol Biol Psychiatry 106:110145
Zaman N, Ahmad H, Ali Abid M, Roome T, Abbas G (2017) Acetaminophen (paracetamol) facilitated extinction lear ning in contextual fear conditioned rats. Lett Drug Des Discovery 14(8):898–903
Zavvari F, Nahavandi A, Goudarzi M (2020) Fluoxetine attenuates stress-induced depressive-like behavior through modulation of hippocampal GAP43 and neurogenesis in male rats. J Chem Neuroanat 103:101711
Zhang X, Gainetdinov RR, Beaulieu J-M, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KRR, Caron MG (2005) Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 45(1):11–16
Author information
Authors and Affiliations
Contributions
Following are the author’s contribution in the submitted work: All experiments were performed by Nadeem. The gene expression and ELISA work was assisted by Shumaila Usman, Irfan Khan and Rehan Imad. The data presentation and statistical analysis was helped by Uzair Nisar. Entire word was conceptualized and supervised by Ghulam Abbas. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University (Approval No. 2019–004).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest / Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nadeem, Usman, S., Imad, R. et al. Pre-weaning fluoxetine exposure caused anti-depressant like behavior at adulthood via perturbing tryptophan metabolism in rats. Metab Brain Dis 37, 1415–1422 (2022). https://doi.org/10.1007/s11011-022-00951-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-00951-4