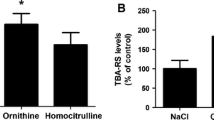
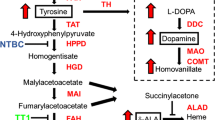
Abstract
Phenylketonuria (PKU) is an inborn error disease in phenylalanine metabolism resulting from defects in the stages of converting phenylalanine to tyrosine. Although the pathophysiology of PKU is not elucidated yet, the toxic effect of phenylalanine on the brain causes severe mental retardation. In relation to learning and memory, the hippocampal PKA / CREB / BDNF pathway may play a role in learning deficits in PKU patients. This study aimed to investigate PKA/CREB/BDNF pathway in hippocampus of chemically induced PKU rats with regard to gender. Sprague-Dawley rat pups were randomized into two groups of both genders. To chemically induce PKU, animals received subcutaneous administration of phenylalanine (5.2 mmol / g) plus p-chlorophenylalanine, phenylalanine hydroxylase inhibitor (0.9 mmol / g); control animals received 0.9% NaCl. Injections started on the 6th day and continued until the 21st day after which locomotor activity, learning and memory were tested. In male PKU rats, locomotor activity was reduced. There were no differences in learning and memory performances of male and female PKU rats. In PKU rats, pCREB / CREB levels in males was unchanged while it decreased in females. Elevated PKA activity, BDNF levels and decreased pCREB/CREB ratio found in female PKU rats were not replicated in PKU males in which BDNF is decreased. Our results display that in this disease model a gender specific differential activation of cAMP/PKA-CREB-BDNF signaling pathway in hippocampus occurs investigation of which can help us to a better understanding of disease pathophysiology.




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available in this published article.
Abbreviations
- PKU:
-
phenylketonuria
- PKA:
-
Protein Kinase A
- BDNF:
-
brain derived neurotrophic factor
- CREB:
-
cAMP response element binding protein
- p-Cl-Phe:
-
p-chloro-phenylalanine
References
Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, Gijselaers HJ et al (2012) Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res 232(2):335–347. https://doi.org/10.1016/j.bbr.2012.03.022
Anderson PJ, Leuzzi V (2010) White matter pathology in phenylketonuria. Mol Genet Metab 99:S3–S9
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110. https://doi.org/10.1007/s10339-011-0430-z
Ardais AP, Rocha AS, Borges MF, Fioreze GT, Sallaberry C, Mioranzza S et al (2016) Caffeine exposure during rat brain development causes memory impairment in a sex selective manner that is offset by caffeine consumption throughout life. Behav Brain Res 303:76–84
Barco A, Bailey CH, Kandel ER (2006) Common molecular mechanisms in explicit and implicit memory. J Neurochem 97(6):1520–1533
Benito E, Barco A (2010) CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33(5):230–240
Berry HK, O'Grady DJ, Perlmutter LJ, Bofinger MK (1979) Intellectual development and academic achievement of children treated early for phenylketonuria. Developmental Medicine & Child Neurology 21(3):311–320
Blau N, van Spronsen FJ, Levy HL (2010) Phenylketonuria. Lancet 376(9750):1417–1427
Bortoluzzi VT, Brust L, Preissler T, de Franceschi ID, Wannmacher CMD (2019) Creatine plus pyruvate supplementation prevents oxidative stress and phosphotransfer network disturbances in the brain of rats subjected to chemically-induced phenylketonuria. Metab Brain Dis 34(6):1649–1660. https://doi.org/10.1007/s11011-019-00472-7
Bronstein PM, Wolkoff FD, Levine MJ (1975) Sex-related differences in rats' open-field activity. Behav Biol
Bruinenberg VM, van der Goot E, van Vliet D, de Groot MJ, Mazzola PN, Heiner-Fokkema MR et al (2016) The behavioral consequence of phenylketonuria in mice depends on the genetic background. Front Behav Neurosci 10:233
Burlina AP, Lachmann RH, Manara R, Cazzorla C, Celato A, van Spronsen FJ, Burlina A (2019) The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: a systematic review. J Inherit Metab Dis 42(2):209–219
Christ SE, Huijbregts SC, de Sonneville LM, White DA (2010) Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol Genet Metab 99:S22–S32
de Groot MJ, Hoeksma M, Reijngoud D-J, de Valk HW, Paans AM, Sauer PJ, van Spronsen FJ (2013) Phenylketonuria: reduced tyrosine brain influx relates to reduced cerebral protein synthesis. Orphanet J Rare Dis 8(1):133
de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C (2009) Global signatures of protein and mRNA expression levels. Mol BioSyst 5(12):1512–1526
Delvalle JA, Dienel G, Greengard O (1978) Comparison of α-methylphenylalanine and p-chlorophenylalanine as inducers of chronic hyperphenylalaninaemia in developing rats. Biochem J 170(3):449–459
Delvalle JA, Greengard O (1976) The regulation of phenylalanine hydroxylase in rat tissues in vivo. The maintenance of high plasma phenylalanine concentrations in suckling rats: a model for phenylketonuria. Biochem J 154(3):613–618
Diamond A, Ciaramitaro V, Donner E, Djali S, Robinson MB (1994) An animal model of early-treated PKU. J Neurosci 14(5):3072–3082
Dienel GA, Cruz NF (2016) Biochemical, metabolic, and behavioral characteristics of immature chronic hyperphenylalanemic rats. Neurochem Res 41(1–2):16–32
Dimer NW, Ferreira BK, Agostini JF, Gomes ML, Kist LW, Malgarin F et al (2018) Brain bioenergetics in rats with acute hyperphenylalaninemia. Neurochem Int 117:188–203. https://doi.org/10.1016/j.neuint.2018.01.001
Enns G, Koch R, Brumm V, Blakely E, Suter R, Jurecki E (2010) Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab 101(2–3):99–109
Fiori E, Oddi D, Ventura R, Colamartino M, Valzania A, D’Amato FR et al (2017) Early-onset behavioral and neurochemical deficits in the genetic mouse model of phenylketonuria. PLoS One 12(8):e0183430
Graves LM, Bornfeldt KE, Raines EW, Potts BC, Macdonald SG, Ross R, Krebs EG (1993) Protein kinase a antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci 90(21):10300–10304
Grimes MT, Harley CW, Darby-King A, McLean JH (2012) PKA increases in the olfactory bulb act as unconditioned stimuli and provide evidence for parallel memory systems: pairing odor with increased PKA creates intermediate-and long-term, but not short-term, memories. Learn Mem 19(3):107–115
Guo J-Q, Deng H-H, Bo X, Yang X-S (2017) Involvement of BDNF/TrkB and ERK/CREB axes in nitroglycerin-induced rat migraine and effects of estrogen on these signals in the migraine. Biology open 6(1):8–16
Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G et al (1998) Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21(4):869–883. https://doi.org/10.1016/S0896-6273(00)80602-9
Jahan S, Singh S, Srivastava A, Kumar V, Kumar D, Pandey A et al (2018) PKA-GSK3β and β-catenin signaling play a critical role in trans-resveratrol mediated neuronal differentiation in human cord blood stem cells. Mol Neurobiol 55(4):2828–2839
Jahja R, Huijbregts SC, de Sonneville LM, van der Meere JJ, Bosch AM, Hollak CE et al (2013) Mental health and social functioning in early treated phenylketonuria: the PKU-COBESO study. Mol Genet Metab 110:S57–S61
Jahja R, van Spronsen FJ, de Sonneville LM, van der Meere JJ, Bosch AM, Hollak CE et al (2016) Social-cognitive functioning and social skills in patients with early treated phenylketonuria: a PKU-COBESO study. J Inherit Metab Dis 39(3):355–362
Jain A, Huang GZ, Woolley CS (2019) Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the Hippocampus. J Neurosci 39(9):1552–1565. https://doi.org/10.1523/jneurosci.1897-18.2018
Janzen D, Nguyen M (2010) Beyond executive function: non-executive cognitive abilities in individuals with PKU. Mol Genet Metab 99:S47–S51
Jiang, H, Zhang, X, Wang, Y, Zhang, H, Li, J, Yang, X, . . . Xu, M (2017) Mechanisms underlying the antidepressant response of acupuncture via PKA/CREB signaling pathway. Neural plasticity, 2017
Kandel ER (2012) The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Molecular brain 5(1):1–12
Konkle AT, McCarthy MM (2011) Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152(1):223–235
Leal G, Bramham C, Duarte C (2017) BDNF and hippocampal synaptic plasticity. In vitamins and hormones, vol 104. Elsevier, pp 153–195
Liu F, Liu Y, Liu J, Ma L-Y (2015) Antenatal taurine improves intrauterine growth-restricted fetal rat brain development which is associated with increasing the activity of PKA-CaMKII/c-fos signal pathway. Neuropediatrics 46(05):299–306
Lu L, Gu X, Li D, Liang L, Zhao Z, Gao J (2011) Mechanisms regulating superoxide generation in experimental models of phenylketonuria: an essential role of NADPH oxidase. Mol Genet Metab 104(3):241–248
Maier T, Güell M, Serrano L (2009) Correlation of mRNA and protein in complex biological samples. FEBS Lett 583(24):3966–3973
Martynyuk A, Glushakov A, Sumners C, Laipis P, Dennis D, Seubert C (2005) Impaired glutamatergic synaptic transmission in the PKU brain. Mol Genet Metab 86:34–42
Nardecchia F, Manti F, Chiarotti F, Carducci C, Carducci C, Leuzzi V (2015) Neurocognitive and neuroimaging outcome of early treated young adult PKU patients: a longitudinal study. Mol Genet Metab 115(2–3):84–90
Ögren SO, Stiedl O (2010) Passive Avoidance. In: Stolerman IP (ed) Encyclopedia of psychopharmacology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 960–967
Parks RJ, Ray G, Bienvenu LA, Rose RA, Howlett SE (2014) Sex differences in SR Ca2+ release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J Mol Cell Cardiol 75:162–173
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(−delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatistics, bioinformatics and biomathematics 3(3):71–85
Rech VC, Feksa LR, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res 27(5):353–357. https://doi.org/10.1023/a:1015529511664
Sakamoto K, Karelina K, Obrietan K (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116(1):1–9
Simon KR, dos Santos RM, Scaini G, Leffa DD, Damiani AP, Furlanetto CB et al (2013) DNA damage induced by phenylalanine and its analogue p-chlorophenylalanine in blood and brain of rats subjected to a model of hyperphenylalaninemia. Biochem Cell Biol 91(5):319–324
Surtees R, Blau N (2000) The neurochemistry of phenylketonuria. Eur J Pediatr 159(2):S109–S113
van Spronsen FJ, van Wegberg AM, Ahring K, Bélanger-Quintana A, Blau N, Bosch AM et al (2017) Key European guidelines for the diagnosis and management of patients with phenylketonuria. The lancet Diabetes & endocrinology 5(9):743–756
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13(4):227–232
Vrolyk V, Haruna J, Benoit-Biancamano MO (2018) Neonatal and juvenile ocular development in Sprague-Dawley rats: a Histomorphological and Immunohistochemical study. Vet Pathol 55(2):310–330. https://doi.org/10.1177/0300985817738098
Zhong Y, Zhu Y, He T, Li W, Yan H, Miao Y (2016) Rolipram-induced improvement of cognitive function correlates with changes in hippocampal CREB phosphorylation, BDNF and arc protein levels. Neurosci Lett 610:171–176
Funding
This work was funded from Hacettepe University Grant No: TDK-2018-16969 to E.B and by TUBİTAK 2214-A International Research Fellowship Program to C.C.
Author information
Authors and Affiliations
Contributions
EB and ÇÇ conceived the study; EB and EEK supervised the study; EB and EEK designed experiments; ÇÇ, PTA and MG performed experiments; EEK, PTA and EB analyzed data; EB, ÇÇ and EEK wrote the manuscript; PTA and MG made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors have nothing to declare.
Ethics approval
All experimental procedures were designed to minimize the number of animals used and their suffering and were approved by Hacettepe University Committee on Animal Ethics (Protocol No 2018/02–12).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Cicek, C., Eren-Koçak, E., Telkoparan-Akillilar, P. et al. cAMP/PKA-CREB-BDNF signaling pathway in hippocampus of rats subjected to chemically-induced phenylketonuria. Metab Brain Dis 37, 545–557 (2022). https://doi.org/10.1007/s11011-021-00865-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00865-7