Abstract
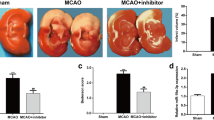
Rodent focal ischemia models are widely used to mimic and examine human strokes. To the best of our knowledge, no investigation has systematically examined the expression changes of microRNA (miR)-449a and Amphiregulin (AREG) as well as their biological relationship during middle cerebral artery occlusion (MCAO) and oxygen and glucose deprivation/reperfusion (OGD/R). The present study examined the histological and behavioral outcomes of MCAO and the function of miR-449a and AREG in cerebral ischemic injury. Rats were subjected to 2 h MCAO, which was followed by reperfusion. miR-449a and AREG were examined in the injury tissues of MCAO rats and the OGD/R cell line by reverse transcription-quantitative polymerase chain reaction. Protein expressions of AREG in the injury tissues of MCAO rats was measured using an immunohistochemistry and the protein expression levels of AREG, epidermal growth factor receptor (EGFR), phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt) and the phosphorylation level of Akt (p-Akt) were analyzed by western blotting. Cell apoptosis was examined following the knock down and subsequent overexpression of AREG in a human OGD/R neuronal cell line by small interfering RNAs (siRNAs) and plasmid transfection. Luciferase reporter assays were used to validate the target of miR-449a. The expression changes and regulatory mechanisms of miR-449a and AREG in an ischemia/reperfusion (I/R) injury model were examined in vivo and in vitro. The neurological deficit score, brain edema volume, cerebral infarct area, and the number of apoptosis cells in ischemic rats were all markedly elevated, than that in the control rats. The expression of miR-449a was decreased and AREG was increased in the MCAO rats and human OGD/R neuronal cell line. miR-449a inhibition or AREG overexpression in OGD/R cells resulted in a significant decrease in apoptotic cells, and AREG was revealed to be one of the direct targets of miR-449a. Molecular recovery was observed following transfection with miR-449a mimics and AREG knockdown in an OGD/R model in vitro. The present study demonstrated that miR-449a was downregulated while AREG was upregulated in cerebral ischemic injury, and the recovery of neurological function can be obtained following the overexpression of miR-449a and the knockdown of AREG in an I/R injury model. miR-449a functions in ischemic stroke via directly targeting AREG. These findings suggest a novel mechanism involving in cerebral I/R injury model and may aid investigators in gaining a deeper understanding of strokes in a clinical setting.






Similar content being viewed by others
References
Ambros V (2004) The functions of animal microRNAs. Nature 431:350
Barca-Mayo O, De Pietri Tonelli D (2014) Convergent microRNA actions coordinate neocortical development. Cell Mol Life Sci 71(16):2975–2995
Berasain C, Avila MA (2014) Amphiregulin. Semin Cell Dev Biol 28:31–41
Besancon E, Guo S, Lok J, Tymianski M, Lo EH (2008) Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29(5):268–275
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40(5):1524–4628 (Electronic)
Chen X, Wu H, Chen H, Wang Q, Xie XJ (2018) Shen JA-Ohoo. Astragaloside VI Promotes Neural Stem Cell Proliferation and Enhances Neurological Function Recovery in Transient Cerebral Ischemic Injury via Activating EGFR/MAPK Signaling Cascades. https://doi.org/10.1007/s12035-018-1294-3
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22(9):391–397
Eckstein N, Servan K, Girard L, Cai D, von Jonquieres G, Jaehde U et al (2008) Epidermal growth factor receptor pathway analysis identifies Amphiregulin as a key factor for cisplatin resistance of human breast Cancer cells. J Biol Chem 283(2):739–750
El-Marasy, SAA-Ohoo, Abdel-Rahman RF, Abd-Elsalam RM. (2018) Neuroprotective effect of vildagliptin against cerebral ischemia in rats. https://doi.org/10.1007/s00210-018-1537-x
Falk A, Frisen J (2002) Amphiregulin is a mitogen for adult neural stem cells. J Neurosci Res 69(6):757–762
Fan J, Chen M, Wang X, Tian Z, Wang J, Fan D et al (2018) Targeting Smox is neuroprotective and ameliorates brain inflammation in cerebral ischemia/reperfusion rats. Toxicol Sci. https://doi.org/10.1093/toxsci/kfy300
Green AR, Shuaib A (2006) Therapeutic strategies for the treatment of stroke. Drug Discov Today 11(15):681–693
Group* TNIoNDaSr-PSS (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24): 1581–1588
Huang Y, Hu ZA-Ohoo (2018) UBIAD1 protects against oxygen-glucose deprivation/reperfusion-induced multiple subcellular organelles injury through PI3K/AKT pathway in N2A cells. J Cell Physiol 233(9):7480–7496. https://doi.org/10.1002/jcp.26602
Kisoh K, Hayashi H, Arai M, Orita M, Yuan B, Takagi NA-Ohoo (2018) Possible Involvement of PI3-K/Akt-Dependent GSK-3beta Signaling in Proliferation of Neural Progenitor Cells After Hypoxic Exposure. https://doi.org/10.1007/s12035-018-1216-4
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are MicroRNA targets. Cell 120(1):15–20
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):0039–2499
Lopez-Morales MA, Castello-Ruiz M, Burguete MC, Jover-Mengual T, Aliena-Valero A, Centeno JM, et al. (2018) Molecular mechanisms underlying the neuroprotective role of atrial natriuretic peptide in experimental acute ischemic stroke
Overgaard K, Sereghy T, Boysen G, Pedersen H, Høyer S, Diemer NH (1992) A rat model of reproducible cerebral infarction using thrombotic blood clot emboli. J Cereb Blood Flow Metab 12(3):484–490
Qi D, Ouyang C, Wang Y, Zhang S, Ma X, Song Y, et al. (2014) HO-1 attenuates hippocampal neurons injury via the activation of BDNF-TrkB-PI3K/Akt signaling pathway in stroke. Brain Res 19(1577):69–76. https://doi.org/10.1016/j.brainres
Qian J, Wang L, Li Q, Sha D, Wang J, Zhang J et al (2019) Ultrasound-targeted microbubble enhances migration and therapeutic efficacy of marrow mesenchymal stem cell on rat middle cerebral artery occlusion stroke model. J Cell Biochem 120(3):3315–3322
Wu J, Bao J, Kim M, Yuan S, Tang C, Zheng H et al (2014) Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci U S A 111(28):E2851–E2857
Yan H, Zhang X, Hu W, Ma J, Hou W, Zhang X, et al. (2014) Histamine H3 receptors aggravate cerebral ischaemic injury by histamine-independent mechanisms. Nat Commun. https://doi.org/10.1038/ncomms4334
Yang J, Xiong LL, Wang YC, He X, Jiang L, Fu SJ et al (2018) Oligodendrocyte precursor cell transplantation promotes functional recovery following contusive spinal cord injury in rats and is associated with altered microRNA expression. Mol Med Rep 17(1):771–782
Yao Y, Ma J, Xue Y, Wang P, Li Z, Li Z et al (2015) MiR-449a exerts tumor-suppressive functions in human glioblastoma by targeting Myc-associated zinc-finger protein. Mol Oncol 9(3):640–656
Yu H, Lu Y, Li Z, Wang Q (2014) microRNA-133: expression, function and therapeutic potential in muscle diseases and cancer. Curr Drug Targets 15(9):817–828
Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR (1997) A rat model of focal embolic cerebral ischemia. Brain Res 766(1):83–92
Zhang G, Zhang JH, Feng J, Li Q, Wu X, Qin X (2011) Electrical stimulation of olfactory bulb downregulates RGMa expression after ischemia/reperfusion injury in rats. Brain Res Bull 86(3–4):254–261
Zhang X, Du Q, Yang Y, Wang J, Liu Y, Zhao Z, et al. (2014) Salidroside alleviates Ischemic Brain Injury in Mice with Ischemic Stroke through regulating BDNK mediated PI3K/Akt Pathway. https://doi.org/10.1016/j.bcp.2018.08.015
Zhou J, Du T, Li B, Rong Y, Verkhratsky A, Peng L. (2015) Crosstalk Between MAPK/ERK and PI3K/AKT Signal Pathways During Brain Ischemia/Reperfusion. https://doi.org/10.1177/1759091415602463
Zhu Q, Enkhjargal B, Huang L, Zhang T, Sun C, Xie Z, et al. (2018) Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-kappaB pathway after subarachnoid hemorrhage in rats. J Neuroinflammation 15(1):178. https://doi.org/10.1186/s12974-018-1211-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Y., Zhang, X., Han, Z. et al. Expression and regulation of miR-449a and AREG in cerebral ischemic injury. Metab Brain Dis 34, 821–832 (2019). https://doi.org/10.1007/s11011-019-0393-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-0393-9