Abstract
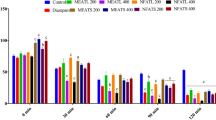
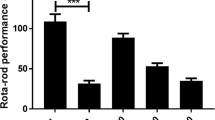
Nardostachys jatamansi has profound applications against pharmacological interventions and is categorized as a hypno-sedative drug according to Ayurveda. In the present study probable mechanism of anxiolytic action of Nardostachys jatamansi extract (NJE) was studied using behavioral anxiolytic tests (Elevated plus maze, Open field test, Light dark box test, and Vogel’s conflict test) in mice. Mice were treated orally with NJE (250 mg/kg) for 3, 7 and 14 days or diazepam (1 mg/kg) followed by behavioral assessment and estimation of monoamine neurotransmitters, GABA, and antioxidant enzymes. Treatment of mice for 7 days caused an increase in time spent in open arms in elevated plus maze, number of line crossings in open field test, increased time spent in lit compartment of light-dark box test, an increase in number of licks made and shocks accepted in Vogel’s conflict test, with results comparable to diazepam and this treatment also caused a significant increase in monoamine neurotransmitters and GABA in brain and tissue antioxidant parameters. Co-treatment of NJE with flumazenil (GABA-benzodiazepine antagonist; 0.5 mg/kg i.p) or picrotoxin (GABAA gated chloride channel blocker; 1 mg/kg i.p) caused a blockage/antagonised anxiolytic actions of NJE by causing a significant reduction in time spent in open arms of elevated plus maze, an decrease in number of line crossing in open field test and also number of shocks and licks accepted in Vogel’s conflict test. Further, NJE was radiolabelled with technetium99m at their hydroxyl groups following which purity as well as in vivo and in vitro stability of radiolabelled formulations was evaluated. The blood kinetics and in vivo bio-distribution studies were carried out in rabbits and mice respectively. Labeled formulation was found to be stable in vitro (96 to 93% stability) and in vivo (96 to 92% stability). The labeled compound was cleared rapidly from blood (within 24 h) and accumulated majorly in kidneys (11.65 ± 1.33), liver (6.07 ± 0.94), and blood (4.03 ± 0.63) after 1 h. However, a small amount was observed in brain (0.1 ± 0.02) probably because of its inability to cross blood-brain barrier. These results highlight biodistribution pattern of NJE, and also indicated that a 7-day treatment with NJE produced significant anxiolytic effects in mice and also a significant increase in brain monoamine and GABA neurotransmitter levels and suggests that anxiolytic effects of NJE are primarily and plausibly mediated by activating GABAergic receptor complex.














Similar content being viewed by others
References
Ahmad M, Yousuf S, Khan MB, Hoda MN, Ahmad AS, Ansari MA, Islam F (2006) Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: behavioral, neurochemical, and immunohistochemical studies. Pharmacol Biochem Behav 83(1):150–160
Alburges ME, Narang N, Wamsley JK (1993) A sensitive and rapid HPLC-ECD method for the simultaneous analysis of norepinephrine, dopamine, serotonin and their primary metabolites in brain tissue. Biomed Chromatogr 7(6):306–310
Ali S, Ansari KA, Jafry MA, Kabeer H, Diwakar G (2000) Nardostachys jatamansi protects against liver damage induced by thioacetamide in rats. J Ethnopharmacol 71(3):359–363
Anuradha H, Srikumar BN, Rao BS, Lakshmana M (2008) Euphorbia hirta reverses chronic stress-induced anxiety and mediates its action through the GABAA receptor benzodiazepine receptor-Cl− channel complex. J Neural Transm 115(1):35–42
Balendiran GK, Dabur R, Fraser D (2004) The role of glutathione in cancer. Cell Biochem Funct 22(6):343–352
Barchas JD, Altemus M (1999) Biochemical hypotheses of mood and anxiety disorders. In: Siegel GJ, Agranoff BW, Albers RW, Fischer SK, Uhler MD (eds) Basic neurochemistry: molecular, cellular and medical aspects. Lipincott-Raven Publishers, Philadelphia, pp 1073–1093
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125(1):141–149
Bonetti EP, Pieri L, Cumin R, Schaffner R, Pieri M, Gamzu ER, Müller RKM, Haefely W (1982) Benzodiazepine antagonist Ro 15-1788: neurological and behavioral effects. Psychopharmacology 78(1):8–18
Bourin M, Hascoët M (2003) The mouse light/dark box test. Eur J Pharmacol 463(1):55–65
Buccafusco JJ (ed) (2000) Methods of behavior analysis in neuroscience. CRC Press
Carro-Juárez M, Rodríguez-Landa JF, de Lourdes Rodríguez-Peña M, de Jesús Rovirosa-Hernández M, García-Orduña F (2012) The aqueous crude extract of Montanoa frutescens produces anxiolytic-like effects similarly to diazepam in Wistar rats: involvement of GABAA receptor. J Ethnopharmacol 143(2):592–598
Cavadas C, Araujo I, Cotrim MD, Amaral T, Cunha AP, Macedo T, Ribeiro CF (1995) In vitro study on the interaction of Valeriana officinalis L. extracts and their amino acids on GABAA receptor in rat brain. Arzneimittelforschung 45(7):753–755
Chouinard G (2004) Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry 65:7–12
Crawley J, Goodwin FK (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13(2):167–170
Dhingra D, Goyal PK (2008) Inhibition of MAO and GABA: probable mechanisms for antidepressant-like activity of Nardostachys jatamansi DC. in mice. Indian J Exp Biol 46(4):212–218
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Ferguson JM (2001) SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry 3(1):22–27
Fernández-Guasti A, Ferreira A, Picazo O (2001) Diazepam, but not buspirone, induces similar anxiolytic-like actions in lactating and ovariectomized Wistar rats. Pharmacol Biochem Behav 70(1):85–93
File SE (1980) The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 2(3):219–238
File SE, Pellow S (1984) The anxiogenic action of Ro 15-1788 is reversed by chronic, but not by acute, treatment with chlordiazepoxide. Brain Res 310(1):154–156
Gonzalez LE, File SE (1997) A five minute experience in the elevated plus-maze alters the state of the benzodiazepine receptor in the dorsal raphe nucleus. J Neurosci 17(4):1505–1511
Gould TD, Dao DT, Kovacsics CE (2009) The open field test. In: Gould TD (ed) Mood and anxiety related phenotypes in mice. Humana Press, New York, pp 1–20
Gupta D, Radhakrishnan M, Kurhe Y (2014) Anxiolytic-like effects of alverine citrate in experimental mouse models of anxiety. Eur J Pharmacol 742:94–101
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Haefely W (1982) Antagonists of benzodiazepines: functional aspects. Adv Biochem Psychopharmacol 38:73–93
Houghton PJ (1999) The scientific basis for the reputed activity of valerian. J Pharm Pharmacol 51(5):505–512
Hunkeler W, Möhler H, Pieri L, Polc P, Bonetti EP, Cumin R, Schaffner R, Haefely W (1981) Selective antagonists of benzodiazepines. Nature 290:514–516
Jadhav VM, Thorat RM, Kadam VJ, Kamble SS (2009) Herbal anxiolyte: Nardostachys jatamansi. J Pharm Res 2(8):1208–1211
Jardim MC, Aguiar DC, Moreira FA, Guimarães FS (2005) Role of glutamate ionotropic and benzodiazepine receptors in the ventromedial hypothalamic nucleus on anxiety. Pharmacol Biochem Behav 82(1):182–189
Jayashree GV, Kumar KH, Krupashree K, Rachitha P, Khanum F (2015) LC–ESI–MS/MS analysis of Asparagus racemosus Willd. roots and its protective effects against t-BHP induced oxidative stress in rats. Ind Crop Prod 78:102–109
Jindal A, Mahesh R, Kumar B (2013) Anxiolytic-like effect of linezolid in experimental mouse models of anxiety. Prog Neuro-Psychopharmacol Biol Psychiatry 40:47–53
Johnston GA, Hanrahan JR, Chebib M, Duke RK, Mewett KN (2006) Modulation of ionotropic GABA receptors by natural products of plant origin. Adv Pharmacol 54:285
Joshi H, Parle M (2006) Nardostachys jatamansi improves learning and memory in mice. J Med Food 9(1):113–118
Jung JW, Yoon BH, Oh HR, Ahn J, Kim SY, Park S, Ryu JH (2006) Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol Pharm Bull 29(2):261–265
Kandikattu HK, Venuprasad MP, Pal A, Khanum F (2014) Phytochemical analysis and exercise enhancing effects of hydroalcoholic extract of Celastrus paniculatus Willd. Ind Crop Prod 55:217–224
Kandikattu HK, Deep SN, Razack S, Amruta N, Prasad D, Khanum F (2017) Hypoxia induced cognitive impairment modulating activity of Cyperus rotundus. Physiol Behav 175:56–65
Krishnamoorthy G, Shabi MM, Ravindhran D, Uthrapathy S, Rajamanickam VG, Dubey GP (2009) Nardostachys jatamansi: cardioprotective and hypolipidemic herb. J Pharm Res 2(4):574–578
Krupashree K, Kumar KH, Rachitha P, Jayashree GV, Khanum F (2014) Chemical composition, antioxidant and macromolecule damage protective effects of Picrorhiza kurroa Royle ex Benth. S Afr J Bot 94:249–254
Kulkarni SK (2005) Handbook of experimental pharmacology, 3rd edn. Vallabh Prakashan, New Delhi
Kumar KH, Razack S, Nallamuthu I, Khanum F (2014) Phytochemical analysis and biological properties of Cyperus rotundus L. Ind Crop Prod 52:815–826
Kumari SN, Madhu LN (2014) Dietary supplementation of natural and synthetic products reduces anxiety in mice against electron beam radiation induced oxidative stress. Nitte University Journal of Health Science 4(3):28
Lin JH, Lu AY (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49(4):403–449
Lister RG (1987) The effects of repeated doses of ethanol on exploration and its habituation. Psychopharmacol 92(1):78–83
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Luck H (1965) Catalase. Methods of enzymatic analysis 885–888
Lyle N, Gomes A, Sur T, Munshi S, Paul S, Chatterjee S, Bhattacharyya D (2009) The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav Brain Res 202(2):285–290
Mansouri MT, Soltani M, Naghizadeh B, Farbood Y, Mashak A, Sarkaki A (2014) A possible mechanism for the anxiolytic-like effect of gallic acid in the rat elevated plus maze. Pharmacol Biochem Behav 117:40–46
Mishra D, Chaturvedi RV, Tripathi SC (1995) The fungitoxic effect of the essential oil of the herb Nardostachys jatamansi DC. Trop Agric 72(1):48–52
Nutt DJ, Malizia AL (2001) New insights into the role of the GABAA-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 179(5):390–396
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Periyakoil VS, Skultety K, Sheikh J (2005) Panic, anxiety, and chronic dyspnea. J Palliat Med 8(2):453–459
Prabhu V, Karanth KS, Rao A (1994) Effects of Nardostachys jatamansi on biogenic amines and inhibitory amino acids in the rat brain. Planta Med 60(2):114–117
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463(1–3):3–33
Rao VS, Rao A, Karanth KS (2005) Anticonvulsant and neurotoxicity profile of Nardostachys jatamansi in rats. J Ethnopharmacol 102(3):351–356
Razack S, Khanum F (2012) Anxiolytic effects of Nardostachys jatamansi DC in mice. Ann Phytomed 1:67–73
Rowley HL, Martin KF, Marsden CA (1995) Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods 57(1):93–99
Salim S, Ahmad M, Zafar KS, Ahmad AS, Islam F (2003) Protective effect of Nardostachys jatamansi in rat cerebral ischemia. Pharmacol Biochem Behav 74(2):481–486
Santos MS, Ferreira F, Faro C, Pires E, Carvalho AP, Cunha AP, Macedo T (1994) The amount of GABA present in aqueous extracts of valerian is sufficient to account for [3H] GABA release in synaptosomes. Planta Med 60(5):475–476
Sigel E, Buhr A (1997) The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18(11):425–429
Smith S, Sharp T (1994) Measurement of GABA in rat brain microdialysates using o-phthaldialdehyde—sulphite derivatization and high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 652(2):228–233
Subashini R, Yogeeta S, Gnanapragasam A, Devaki T (2006) Protective effect of Nardostachys jatamansi on oxidative injury and cellular abnormalities during doxorubicin-induced cardiac damage in rats. J Pharm Pharmacol 58(2):257–262
Subashini R, Gnanapragasam A, Senthilkumar S, Yogeeta SK, Devaki T (2007) Protective efficacy of Nardostachys jatamansi (Rhizomes) on mitochondrial respiration and lysosomal hydrolases during doxorubicin induced myocardial injury in rats. J Health Sci 53(1):67–76
Uzun S, Kozumplik O, Jakovljević M, Sedić B (2010) Side effects of treatment with benzodiazepines. Psychiatr Danub 22(1):90–93
Venuprasad MP, Kandikattu HK, Razack S, Khanum F (2014) Phytochemical analysis of Ocimum gratissimum by LC-ESI–MS/MS and its antioxidant and anxiolytic effects. S Afr J Bot 92:151–158
Venuprasad MP, Kandikattu HK, Razack S, Amruta N, Khanum F (2017) Chemical composition of Ocimum sanctum by LC-ESI–MS/MS analysis and its protective effects against smoke induced lung and neuronal tissue damage in rats. Biomed Pharmacother 91:1–2
Wagner JA, Katz RJ (1984) Anxiogenic action of benzodiazepine antagonists Ro 15-1788 and CGS 8216 in the rat. Neurosci Lett 48(3):317–320
Walf AA, Frye CA (2011) The vogel punished drinking task as a bioassay of anxiety-like behavior of mice. In: Mood and anxiety related phenotypes in mice. Humana Press, New York, pp 143–158
Weeks BS (2009) Formulations of dietary supplements and herbal extracts for relaxation and anxiolytic action: Relarian. Med Sci Monit 15(11):256–262
Wiart C (2007) Ethnopharmacology of medicinal plants Asia and the Pacific. Humana Press Inc, Totowa
Williams CL, Stancel GM (1990) Antiviral agents. In: Gilman AG, Goodman LS, Rall TW, Murad F (eds) The Pharmacological Basis of Therapeutics. Macmillan Publishing Co, New York, pp 1205
Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD (2004) Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav 78(3):445–458
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Razack, S., Kandikattu, H.K., Venuprasad, M.P. et al. Anxiolytic actions of Nardostachys jatamansi via GABA benzodiazepine channel complex mechanism and its biodistribution studies. Metab Brain Dis 33, 1533–1549 (2018). https://doi.org/10.1007/s11011-018-0261-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0261-z