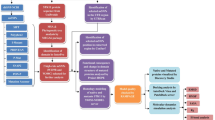
Abstract
The NF1 gene encodes for neurofibromin protein, which is ubiquitously expressed, but most highly in the central nervous system. Non-synonymous SNPs (nsSNPs) in the NF1 gene were found to be associated with Neurofibromatosis Type 1 disease, which is characterized by the growth of tumors along nerves in the skin, brain, and other parts of the body. In this study, we used several in silico predictions tools to analyze 16 nsSNPs in the RAS-GAP domain of neurofibromin, the K1444N (K1423N) mutation was predicted as the most pathogenic. The comparative molecular dynamic simulation (MDS; 50 ns) between the wild type and the K1444N (K1423N) mutant suggested a significant change in the electrostatic potential. In addition, the RMSD, RMSF, Rg, hydrogen bonds, and PCA analysis confirmed the loss of flexibility and increase in compactness of the mutant protein. Further, SASA analysis revealed exchange between hydrophobic and hydrophilic residues from the core of the RAS-GAP domain to the surface of the mutant domain, consistent with the secondary structure analysis that showed significant alteration in the mutant protein conformation. Our data concludes that the K1444N (K1423N) mutant lead to increasing the rigidity and compactness of the protein. This study provides evidence of the benefits of the computational tools in predicting the pathogenicity of genetic mutations and suggests the application of MDS and different in silico prediction tools for variant assessment and classification in genetic clinics.






Similar content being viewed by others
References
Abdel-Azeim S, Oliva R, Chermak E et al (2014) Molecular dynamics characterization of five pathogenic factor X mutants associated with decreased catalytic activity. Biochemistry 53:6992–7001. https://doi.org/10.1021/bi500770p
Acharya V, Nagarajaram HA (2012) Hansa: an automated method for discriminating disease and neutral human nsSNPs. Hum Mutat 33:332–337. https://doi.org/10.1002/humu.21642
Adjei AA (2001) Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst 93:1062–1074. https://doi.org/10.1016/S0378-4274(02)00422-8
Adzhubei IA, Schmidt S, Peshkin L et al (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Agrahari A, George Priya Doss C (2015) Impact of I30T and I30M substitution in MPZ gene associated with Dejerine-Sottas syndrome type B (DSSB): a molecular modeling and dynamics. J Theor Biol 382:23–33. https://doi.org/10.1016/j.jtbi.2015.06.019
Agrahari AK, Sneha P, George Priya Doss C et al (2017) A profound computational study to prioritize the disease-causing mutations in PRPS1 gene. Metab Brain Dis 33:589–600. https://doi.org/10.1007/s11011-017-0121-2
Agrahari AK, Kumar A, Siva R et al (2018) Substitution impact of highly conserved arginine residue at position 75 in GJB1 gene in association with X-linked Charcot–Marie-tooth disease: a computational study. J Theor Biol 437:305–317. https://doi.org/10.1016/j.jtbi.2017.10.028
Ahmadian MR, Kiel C, Stege P, Scheffzek K (2003) Structural fingerprints of the Ras-GTPase activating proteins neurofibromin and p120GAP. J Mol Biol 329:699–710. https://doi.org/10.1016/S0022-2836(03)00514-X
Ali SK, Sneha P, Priyadharshini Christy J et al (2017) Molecular dynamics-based analyses of the structural instability and secondary structure of the fibrinogen gamma chain protein with the D356V mutation. J Biomol Struct Dyn 35:2714–2724. https://doi.org/10.1080/07391102.2016.1229634
Andersen LB, Ballester R, Marchuk DA et al (1993) A conserved alternative splice in the von Recklinghausen neurofibromatosis (NF1) gene produces two neurofibromin isoforms, both of which have GTPase-activating protein activity. Mol Cell Biol 13:487–495. https://doi.org/10.1128/MCB.13.1.487.Updated
Ars E, Kruyer H, Morell M et al (2003) Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients. J Med Genet 40:e82. https://doi.org/10.1136/JMG.40.6.E82
Arun D, Gutmann DH (2004) Recent advances in neurofibromatosis type 1. Curr Opin Neurol 17:101–105
Ashkenazy H, Erez E, Martz E et al (2010) ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38:W529–W533. https://doi.org/10.1093/nar/gkq399
Bairoch A, Apweiler R (1996) The SWISS-PROT protein sequence data bank and its new supplement TREMBL. Nucleic Acids Res 24:21–25. https://doi.org/10.1093/nar/24.1.21
Ballester R, Marchuk D, Boguski M et al (1990) The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell 63:851–859. https://doi.org/10.1016/0092-8674(90)90151-4
Bendl J, Stourac J, Salanda O et al (2014) PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput Biol 10. https://doi.org/10.1371/journal.pcbi.1003440
Benz RW, Castro-Román F, Tobias DJ, White SH (2005) Experimental validation of molecular dynamics simulations of lipid bilayers: a new approach. Biophys J 88:805–817. https://doi.org/10.1529/biophysj.104.046821
Bromberg Y, Rost B (2007) SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res 35:3823–3835. https://doi.org/10.1093/nar/gkm238
Capriotti E, Calabrese R, Casadio R (2006) Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics 22:2729–2734. https://doi.org/10.1093/bioinformatics/btl423
Capriotti E, Fariselli P, Rossi I, Casadio R (2008) A three-state prediction of single point mutations on protein stability changes. BMC Bioinformatics 9:S6. https://doi.org/10.1186/1471-2105-9-S2-S6
Capriotti E, Altman RB, Bromberg Y (2013) Collective judgment predicts disease-associated single nucleotide variants. BMC Genomics 14(Suppl 3):S2. https://doi.org/10.1186/1471-2164-14-S3-S2
Chakraborty S (2012) Enumerating pathways of proton abstraction based on a spatial and electrostatic analysis of residues in the catalytic site. PLoS One 7. https://doi.org/10.1371/journal.pone.0039577
Chakraborty S, Minda R, Salaye L et al (2011) Active site detection by spatial conformity and electrostatic analysis-unravelling a proteolytic function in shrimp alkaline phosphatase. PLoS One 6:e28470. https://doi.org/10.1371/journal.pone.0028470
Chen B, Li L, Ren W et al (2017) A novel missense mutation in the ALPL gene causes dysfunction of the protein. Mol Med Rep 16:710–718. https://doi.org/10.3892/mmr.2017.6668
Chin SL, Lu Q, Dane EL et al (2016) Combined molecular dynamics simulations and experimental studies of the structure and dynamics of poly-amido-saccharides. J Am Chem Soc 138:6532–6540. https://doi.org/10.1021/jacs.6b01837
Cichowski K, Jacks T (2001) NF1 tumor suppressor gene function: narrowing the GAP. Cell 104:593–604
Cruz FJ, Lopes JN, Calado JC, Minas da Piedade ME (2005) A molecular dynamics study of the thermodynamic properties of calcium apatites. 1. Hexagonal phases. J Phys Chem B 109:24473–24479. https://doi.org/10.1021/jp054304p
DeClue JE, Cohen BD, Lowy DR (1991) Identification and characterization of the neurofibromatosis type 1 protein product. Proc Natl Acad Sci U S A 88:9914–9918. https://doi.org/10.1073/pnas.88.22.9914
Doss CGP, Sethumadhavan R (2009) Investigation on the role of nsSNPs in HNPCC genes--a bioinformatics approach. J Biomed Sci 16:42. https://doi.org/10.1186/1423-0127-16-42
Doss CGP, Chakraborty C, Chen L, Zhu H (2014) Integrating in silico prediction methods, molecular docking, and molecular dynamics simulation to predict the impact of ALK missense mutations in structural perspective. Biomed Res Int 2014:895831–895814. https://doi.org/10.1155/2014/895831
Doss CGP, Alasmar DR, Bux RI et al (2016) Genetic epidemiology of Glucose-6-dehydrogenase deficiency in the Arab world. Sci Rep 6. https://doi.org/10.1038/srep37284
Dunzendorfer-Matt T, Mercado EL, Maly K et al (2016) The neurofibromin recruitment factor Spred1 binds to the GAP related domain without affecting Ras inactivation. Proc Natl Acad Sci 113:7497–7502. https://doi.org/10.1073/pnas.1607298113
Efimov AV, Brazhnikov EV (2003) Relationship between intramolecular hydrogen bonding and solvent accessibility of side-chain donors and acceptors in proteins. FEBS Lett 554:389–393. https://doi.org/10.1016/S0014-5793(03)01189-X
Elber R (2016) Perspective: computer simulations of long time dynamics. J Chem Phys 144:060901. https://doi.org/10.1063/1.4940794
Emmerich D, Zemojtel T, Hecht J et al (2015) Somatic neurofibromatosis type 1 (NF1) inactivation events in cutaneous neurofibromas of a single NF1 patient. Eur J Hum Genet 23:870–873. https://doi.org/10.1038/ejhg.2014.210
Eswar N, Webb B, Marti-Renom MA, et al (2007) Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci Chapter 2:Unit 2.9. https://doi.org/10.1002/0471140864.ps0209s50
Gottfried ON, Viskochil DH, Fults DW, Couldwell WT (2006) Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery 58:1–16
Grantham R (1974) Amino acid difference formula to help explain protein evolution. Science 185:862–864. https://doi.org/10.1126/science.185.4154.862
Guex N, Peitsch MC, Schwede T (2009) Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30(Suppl 1):S162–S173. https://doi.org/10.1002/elps.200900140
Gutmann DH, Boguski M, Marchuk D et al (1993) Analysis of the neurofibromatosis type 1 (NF1) GAP-related domain by site-directed mutagenesis. Oncogene 8:761–769
Hess B, Kutzner C, Van Der Spoel D, Lindahl E (2008) GRGMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447. https://doi.org/10.1021/ct700301q
Hsueh Y-P (2012) From neurodevelopment to neurodegeneration: the interaction of neurofibromin and valosin-containing protein/p97 in regulation of dendritic spine formation. J Biomed Sci 19:33. https://doi.org/10.1186/1423-0127-19-33
Hubbard RE, Kamran Haider M (2010) Hydrogen bonds in proteins: role and strength. In: Encyclopedia of life sciences. John Wiley & Sons, Ltd, Chichester
Huson SM, Compston DA, Clark P, Harper PS (1989) A genetic study of von Recklinghausen neurofibromatosis in south East Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet 26:704–711
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637. https://doi.org/10.1002/bip.360221211
Kim S, Jhong J-H, Lee J, Koo J-Y (2017) Meta-analytic support vector machine for integrating multiple omics data. BioData Min 10:2. https://doi.org/10.1186/s13040-017-0126-8
Kiuru M, Busam KJ (2017) The NF1 gene in tumor syndromes and melanoma. Lab Investig 97:146–157
Klose A, Ahmadian MR, Schuelke M et al (1998) Selective disactivation of neurofibromin GAP activity in neurofibromatosis type 1. Hum Mol Genet 7:1261–1268. https://doi.org/10.1093/hmg/7.8.1261
Krawczak M, Ball EV, Fenton I et al (2000) Human gene mutation database-a biomedical information and research resource. Hum Mutat 15:45–51. https://doi.org/10.1002/(SICI)1098-1004(200001)15:1<45::AID-HUMU10>3.0.CO;2-T
Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4:1073–1082. https://doi.org/10.1038/nprot.2009.86
Kumar A, Pintus F, Di Petrillo A et al (2018) Novel 2-pheynlbenzofuran derivatives as selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Sci Rep 8:4424. https://doi.org/10.1038/s41598-018-22747-2
Li Y, Bollag G, Clark R et al (1992) Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell 69:275–281. https://doi.org/10.1016/0092-8674(92)90408-5
Li C, Medvedev GA, Lee E-W et al (2012) Molecular dynamics simulations and experimental studies of the thermomechanical response of an epoxy thermoset polymer. Polymer (Guildf) 53:4222–4230. https://doi.org/10.1016/J.POLYMER.2012.07.026
Lovell SC, Davis IW, Arendall WB et al (2003) tructure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins 50:437–450. https://doi.org/10.1002/prot.10286
Marrone A, Re N, Storchi L (2016) The effects of Ca2+ concentration and E200K mutation on the aggregation propensity of PrPC: a computational study. PLoS One 11:e0168039. https://doi.org/10.1371/journal.pone.0168039
Martin GA, Viskoohil D, Bollag G et al (1990) The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell 63:843–849. https://doi.org/10.1016/0092-8674(90)90150-D
McCulloch DG, Marks NA, McKenzie DR, Prawer S (1995) Molecular dynamics and experimental studies of preferred orientation induced by compressive stress. Nucl Inst Methods Phys Res B 106:545–549. https://doi.org/10.1016/0168-583X(95)00767-9
Morcos P, Thapar N, Tusneem N et al (1996) Identification of Neurofibromin mutants that exhibit allele specificity or increased Ras affinity resulting in suppression of activated ras alleles. Mol Cell Biol 16:2496–2503. https://doi.org/10.1128/MCB.16.5.2496
Morgan KJ, Rowley MA, Wiesner SM et al (2007) The GAP-related domain of neurofibromin attenuates proliferation and downregulates N- and K-Ras activation in Nf1-negative AML cells. Leuk Res 31:1115–1121. https://doi.org/10.1016/j.leukres.2006.12.015
Mosaeilhy A, Mohamed MM, George Priya Doss C et al (2017) Genotype-phenotype correlation in 18 Egyptian patients with glutaric acidemia type I. Metab Brain Dis 32:1417–1426. https://doi.org/10.1007/s11011-017-0006-4
Nagarajan R, Chothani SP, Ramakrishnan C et al (2015) Structure based approach for understanding organism specific recognition of protein-RNA complexes. Biol Direct 10. https://doi.org/10.1186/s13062-015-0039-8
Olatubosun A, Väliaho J, Härkönen J et al (2012) PON-P: integrated predictor for pathogenicity of missense variants. Hum Mutat 33:1166–1174. https://doi.org/10.1002/humu.22102
Peltonen S, Kallionpää RA, Peltonen J (2017) Neurofibromatosis type 1 ( NF1 ) gene: Beyond café au lait spots and dermal neurofibromas. Exp Dermatol 26:645–648. https://doi.org/10.1111/exd.13212
Poullet P, Lin B, Esson K, Tamanoi F (1994) Functional significance of lysine 1423 of neurofibromin and characterization of a second site suppressor which rescues mutations at this residue and suppresses RAS2Val-19-activated phenotypes. Mol Cell Biol 14:815–821. https://doi.org/10.1128/MCB.14.1.815
Rajasekaran R, Sudandiradoss C, Doss CGP, Sethumadhavan R (2007) Identification and in silico analysis of functional SNPs of the BRCA1 gene. Genomics 90:447–452. https://doi.org/10.1016/j.ygeno.2007.07.004
Ratner N, Miller SJ (2015) A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer 15:290–301
Réblová K, Kulhánek P, Fajkusová L (2015) Computational study of missense mutations in phenylalanine hydroxylase. J Mol Model 21:70. https://doi.org/10.1007/s00894-015-2620-6
Resat H, Straatsma TP, Dixon DA, Miller JH (2001) The arginine finger of RasGAP helps Gln-61 align the nucleophilic water in GAP-stimulated hydrolysis of GTP. Proc Natl Acad Sci U S A 98:6033–6038. https://doi.org/10.1073/pnas.091506998
Sanjeev A, Mattaparthi VSK (2017) Computational investigation on the effects of H50Q and G51D mutations on the α-Synuclein aggregation propensity. J Biomol Struct Dyn 10:1–13
Scheffzek K, Lautwein A, Kabscht W et al (1996) Crystal structure of the GTPase-activating domain of human p120GAP and implications for the interaction with Ras. Nature 384:591–596. https://doi.org/10.1038/384591a0
Scheffzek K, Ahmadian MR, Kabsch W et al (1997) The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277:333–338. https://doi.org/10.1126/science.277.5324.333
Scheffzek K, Ahmadian MR, Wiesmüller L et al (1998) Structural analysis of the GAP-related domain from neurofibromin and its implications. EMBO J 17:4313–4327. https://doi.org/10.1093/emboj/17.15.4313
Sen S, Goluguri RR, Udgaonkar JB (2017) A dry transition state more compact than the native state is stabilized by non-native interactions during the unfolding of a small protein. Biochemistry 56:3699–3703. https://doi.org/10.1021/acs.biochem.7b00388
Sherry ST (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 29:308–311. https://doi.org/10.1093/nar/29.1.308
Shihab HA, Gough J, Cooper DN et al (2013) Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 34:57–65. https://doi.org/10.1002/humu.22225
Skinner RH, Bradley S, Brown AL et al (1991) Use of the Glu-Glu-Phe C-terminal epitope for rapid purification of the catalytic domain of normal and mutant ras GTPase-activating proteins. J Biol Chem 266:14163–14166
Sneha P, George Priya Doss C (2016) Chapter seven – Molecular dynamics: New frontier in personalized medicine. In: Advances in protein chemistry and structural biology. 102:181–224
Sohier P, Luscan A, Lloyd A et al (2017) Confirmation of mutation landscape of NF1-associated malignant peripheral nerve sheath tumors. Genes Chromosom Cancer 56:421–426. https://doi.org/10.1002/gcc.22446
Srinivasan E, Rajasekaran R (2017) Exploring the cause of aggregation and reduced Zn binding affinity by G85R mutation in SOD1 rendering amyotrophic lateral sclerosis. Proteins Struct Funct Bioinforma 85:1276–1286. https://doi.org/10.1002/prot.25288
Tang H, Thomas PD (2016) Tools for predicting the functional impact of nonsynonymous genetic variation. Genetics 203:635–647
Tavtigian SV, Deffenbaugh AM, Yin L et al (2006) Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet 43:295–305. https://doi.org/10.1136/jmg.2005.033878
Teilum K, Olsen JG, Kragelund BB (2011) Protein stability, flexibility and function. Biochim Biophys Acta (BBA) - Proteins Proteomics 1814:969–976. https://doi.org/10.1016/j.bbapap.2010.11.005
Teng S, Srivastava AK, Wang L (2010) Sequence feature-based prediction of protein stability changes upon amino acid substitutions. BMC Genomics 11:S5. https://doi.org/10.1186/1471-2164-11-S2-S5
Theobald DL, Wuttke DS (2008) Accurate structural correlations from maximum likelihood superpositions. PLoS Comput Biol 4:e43. https://doi.org/10.1371/journal.pcbi.0040043
Theos A, Korf BR (2006) Pathophysiology of neurofibromatosis type 1. Ann Intern Med 144:842–849
Thirumal Kumar D, George Priya Doss C (2017) Role of E542 and E545 missense mutations of PIK3CA in breast cancer: a comparative computational approach. J Biomol Struct Dyn 35:2745–2757. https://doi.org/10.1080/07391102.2016.1231082
Thirumal Kumar D, George Priya Doss C, Sneha P et al (2017) Influence of V54M mutation in giant muscle protein titin: a computational screening and molecular dynamics approach. J Biomol Struct Dyn 35:917–928. https://doi.org/10.1080/07391102.2016.1166456
Tina KG, Bhadra R, Srinivasan N (2007) PIC: Protein Interactions Calculator. Nucleic Acids Res 35:W473–W476. https://doi.org/10.1093/nar/gkm423
Upadhyaya M (2010) Neurofibromatosis type 1: diagnosis and recent advances. Expert Opin Med Diagn 4:307–322. https://doi.org/10.1517/17530059.2010.494660
Upadhyaya M, Osborn MJ, Maynard J et al (1997) Mutational and functional analysis of the neurofibromatosis type 1 (NF1) gene. Hum Genet 99:88–92
Wakui N, Yoshino R, Yasuo N et al (2018) Exploring the selectivity of inhibitor complexes with Bcl-2 and Bcl-XL: a molecular dynamics simulation approach. J Mol Graph Model 79:166–174. https://doi.org/10.1016/j.jmgm.2017.11.011
Welti S, D’Angelo I, Scheffzek K (2008) Structure and function of neurofibromin. In: Neurofibromatoses. KARGER, Basel, 16:113–128
Wiesmuller L, Wittinghofer A (1992) Expression of the GTPase activating domain of the neurofibromatosis type 1 (NF1) gene in Escherichia coli and role of the conserved lysine residue. J Biol Chem 267:10207–10210
Xu GF, O’Connell P, Viskochil D et al (1990) The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 62:599–608. https://doi.org/10.1016/0092-8674(90)90024-9
Xu J, Ren Y, Ge W et al (2010) Molecular dynamics simulation of macromolecules using graphics processing unit. Mol Simul 36:1131–1140. https://doi.org/10.1080/08927022.2010.506512
Yunoue S, Tokuo H, Fukunaga K et al (2003) Neurofibromatosis type I tumor suppressor neurofibromin regulates neuronal differentiation via its GTPase-activating protein function toward Ras. J Biol Chem 278:26958–26969. https://doi.org/10.1074/jbc.M209413200
Zaki OK, Krishnamoorthy N, El Abd HS et al (2017) Two patients with Canavan disease and structural modeling of a novel mutation. Metab Brain Dis 32:171–177. https://doi.org/10.1007/s11011-016-9896-9
Acknowledgements
I would like to thank VIT management for providing the facility, seed money, and platform to carry out the research. No conflict of interest exists.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 128 kb)
Supplementary Fig. 1
Ramachandran plot of native model RAS-GAP domain of NF1 protein obtained by RAMPAGE server. (DOCX 83 kb)
Supplementary Fig. 2
Conservational analysis of residues of RAS-GAP domain of NF1 protein using the ConSurf server. (DOCX 948 kb)
Supplementary Fig. 3
RMSD plot of RAS-GAP domain of NF1 protein (a) first run (b) second run (c) third run of 50 ns of simulation. (DOCX 99 kb)
Supplementary Fig. 4
Analysis of secondary structure element contribution in native and K1444N mutant. (DOCX 15 kb)
Supplementary Fig. 5
Illustration of an explicit change in secondary structure conformation in native (blue) and mutant K1444N mutant (green) RAS-GAP domain of NF1 protein (a) 0 ns (b) 40 ns (c) 50 ns. Color magenta and red are representing the residue region in between 60 and 68 of native and mutant respectively, color yellow and cyan are representing the residue region in between 110 and 120 of native and mutant respectively. (DOCX 1009 kb)
Rights and permissions
About this article
Cite this article
Agrahari, A.K., Muskan, M., George Priya Doss, C. et al. Computational insights of K1444N substitution in GAP-related domain of NF1 gene associated with neurofibromatosis type 1 disease: a molecular modeling and dynamics approach. Metab Brain Dis 33, 1443–1457 (2018). https://doi.org/10.1007/s11011-018-0251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0251-1