Abstract
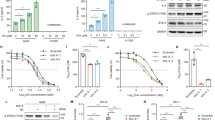
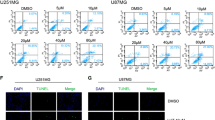
Maintaining genomic integrity is essential for cell survival and viability. Reactive oxygen species (ROS) overproduction results in oxidative stress leading to the genomic instability via generation of small base lesions in DNA and these unrepaired DNA damages lead to various cellular consequences including cancer. Recent data support the concept “oxidative stress is an indispensable participant in fostering proliferation, survival, and migration” in various cancer cell types including glioblastoma cells. In this study we demonstrate that treatment of non-cytotoxic doses of oxidants such as amyloid beta [Aβ(25–35)] peptide, glucose oxidase (GO), and hydrogen peroxide (H2O2) for 24 h and 48 h time points found to increase the expression level and activity of a multifunctional enzyme Apurinic/apyrimidinic endonuclease (APE1), a key enzyme of base excision repair (BER) pathway which takes care of base damages; and also resulted in modulation in the expression levels of downstream BER-pathway enzymes viz. PARP-1, XRCC1, DNA polβ, and ligase IIIα was observed upon oxidative stress in C6 and U-87 MG cells. Oxidants treatment to the C6 and U-87 MG cells also resulted in an elevation in the intracellular expression of glycolytic pathway enzyme Pyruvate kinase M2 (PKM2) and the metastasis inducer protein Ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) as analyzed using Western blotting and Immunofluorescence microscopic studies. Our study also reports that oxidative stress induced for 24 h and 48 h in C6 and U-87 MG cells resulted in extracellular secretion of APE1 and ENPP2 as analyzed using Western blotting in conditioned media. However, the biological significance of extracellular secreted APE1 remains elusive. Oxidative stress also elevated the ENPP2’s LysoPLD activity in conditioned media of C6 and U-87 MG cells. Our results also demonstrate that oxidative stress affects the expression level and localization of APE1, PKM2, and ENPP2 in C6 and U-87 MG cells. As evidenced by the colocalization pattern at 24 h and 48 h time points, it can be attributed that oxidative stress mediates crosstalk between APE1, PKM2, and ENPP2. In addition, when C6 and U-87 MG cells were treated with lysophosphatidic acid (LPA), a bioactive lipid that negatively regulates ENPP2’s LysoPLD activity at 10 μM concentration, demonstrated strong migratory potential in C6 and U-87 MG cells, and also induced migration upon oxidative stress. Altogether, the findings demonstrate the potential of C6 and U-87 MG cells to utilize three proteins viz. APE1, PKM2, and ENPP2 towards migration and survival of gliomas. Thus the knowledge on oxidative stress induced APE1’s interaction with PKM2 and ENPP2 opens a new channel for the therapeutic target(s) for gliomas.














Similar content being viewed by others
References
Alía M, Ramos S, Mateos R, Granado-Serrano AB, Bravo L, Goya L (2006) Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol Appl Pharmacol 212:110–118
Awada R, Rondeau P, Grès S, Saulnier-Blache JS, d'Hellencourt CL, Bourdon E (2012) Autotaxin protects microglial cells against oxidative stress. Free Radic Biol Med 52:516–526
Babu R, Komisarow JM, Agarwal VJ, Rahimpour S, Iyer A, Britt D, Karikari IO, Grossi PM, Thomas S, Friedman AH (2016) Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg 124:998–1007
Benesch MG, Ko YM, McMullen TP, Brindley DN (2014) Autotaxin in the crosshairs: taking aim at cancer and other inflammatory conditions. FEBS Lett 588:2712–2727
Benesch MG, Zhao YY, Curtis JM, McMullen TP, Brindley DN (2015) Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J Lipid Res 56:1134–1144
Bhakat K, Mantha A, Mitra S (2009) Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/ref-1), an essential multifunctional protein. Antioxid Redox Signal 11:621–637
Bobola MS, Blank A, Berger MS, Stevens BA, Silber JR (2001) Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin Cancer Res 7:3510–3518
Capdevila C, Rodriguez Vazquez L, Marti J (2017) Glioblastoma Multiforme and adult neurogenesis in the ventricular-subventricular zone: a review. J Cell Physiol 232:1596–1601
Chaneton B, Gottlieb E (2012) Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci 37:309–316
Chen DS, Herman T, Demple B (1991) Two distinct human DNA diesterases that hydrolyze 3′-blocking deoxyribose fragments from oxidized DNA. Nucleic Acids Res 19:5907–5914
Choi S, Lee YR, Park MS, Joo HK, Cho EJ, Kim HS, Kim CS, Park JB, Irani K, Jeon BH (2013) Histone deacetylases inhibitor trichostatin a modulates the extracellular release of APE1/ref-1. Biochem Biophys Res Commun 435:403–407
Choi E-O, Jeong J-W, Park C, Hong SH, Kim G-Y, Hwang H-J, Cho E-J, Choi YH (2016) Baicalein protects C6 glial cells against hydrogen peroxide-induced oxidative stress and apoptosis through regulation of the Nrf2 signaling pathway. Int J Mol Med 37:798–806
Cholia RP, Kumari S, Kumar S, Kaur M, Kaur M, Kumar R, Dhiman M, Mantha AK (2017) An in vitro study ascertaining the role of H2O2 and glucose oxidase in modulation of antioxidant potential and cancer cell survival mechanisms in glioblastoma U-87 MG cells. Metab Brain Dis 32:1705–1716
Cortés-Cros M, Hemmerlin C, Ferretti S, Zhang J, Gounarides JS, Yin H, Muller A, Haberkorn A, Chene P, Sellers WR (2013) M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth. Proc Natl Acad Sci 110:489–494
Costa B, Bendinelli S, Gabelloni P, Da Pozzo E, Daniele S, Scatena F, Vanacore R, Campiglia P, Bertamino A, Gomez-Monterrey I (2013) Human glioblastoma multiforme: p53 reactivation by a novel MDM2 inhibitor. PLoS One 8:e72281
Dai R, Zhang S (2009) Expressions and their significance of PTTG and PCNA proteins in glioma. Chin-Ger J Clin Oncol 8:110–113
Desai S, Ding M, Wang B, Lu Z, Zhao Q, Shaw K, Yung WA, Weinstein JN, Tan M, Yao J (2014) Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget 5:8202
Desmaret S, Qian L, Vanloo B, Meerschaert K, Van Damme J, Grooten J, Vandekerckhove J, Prestwich GD, Gettemans J (2005) Lysophosphatidic acid affinity chromatography reveals pyruvate kinase as a specific LPA-binding protein. Biol Chem 386:1137–1147
Dhiman M, Zago MP, Nunez S, Amoroso A, Rementeria H, Dousset P, Nunez Burgos F, Garg NJ (2012) Cardiac-oxidized antigens are targets of immune recognition by antibodies and potential molecular determinants in chagas disease pathogenesis. PLoS One 7(1):e28449
Duthie S, Collins A, Duthie G, Dobson V (1997) Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidised pyrimidines) in human lymphocytes. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 393:223–231
Fukushima N, Weiner JA, Chun J (2000) Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev Biol 228:6–18
Harris I, McCracken S, Mak TW (2012) PKM2: a gatekeeper between growth and survival. Cell Res 22:447–449
Hausmann J, Perrakis A, Moolenaar WH (2013) Structure-function relationships of autotaxin, a secreted lysophospholipase D. Advances in Biological Regulation 53:112–117
Hoelzinger DB, Nakada M, Demuth T, Rosensteel T, Reavie LB, Berens ME (2008) Autotaxin: a secreted autocrine/paracrine factor that promotes glioma invasion. J Neuro-Oncol 86:297–309
Hu Y-L, Tee M-K, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, Jaffe RB (2001) Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst 93:762–767
Johannessen T-CA, Bjerkvig R (2012) Molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev Anticancer Ther 12:635–642
Katsifa A, Kaffe E, Nikolaidou-Katsaridou N, Economides AN, Newbigging S, McKerlie C, Aidinis V (2015) The bulk of autotaxin activity is dispensable for adult mouse life. PLoS One 10:e0143083
Kelman Z (1997) PCNA: structure, functions and interactions. Oncogene 14:629–640
Kishi Y, Okudaira S, Tanaka M, Hama K, Shida D, Kitayama J, Yamori T, Aoki J, Fujimaki T, Arai H (2006) Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J Biol Chem 281:17492–17500
Lee YR, Kim KM, Jeon BH, Choi S (2015) Extracellularly secreted APE1/ref-1 triggers apoptosis in triple-negative breast cancer cells via RAGE binding, which is mediated through acetylation. Oncotarget 6:23383
Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P (2017) Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res 27:329–351
Mantha AK, Dhiman M, Taglialatela G, Perez-Polo RJ, Mitra S (2012) Proteomic study of amyloid beta (25–35) peptide exposure to neuronal cells: impact on APE1/ref-1's protein–protein interaction. J Neurosci Res 90:1230–1239
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43:969–980
Mazurek, S., Boschek, C. B., Hugo, F., & Eigenbrodt, E. (2005). Pyruvate kinase type M2 and its role in tumor growth and spreading. Paper presented at the Seminars in cancer biology
Montaldi AP, Godoy PR, Sakamoto-Hojo ET (2015) APE1/REF-1 down-regulation enhances the cytotoxic effects of temozolomide in a resistant glioblastoma cell line. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 793:19–29
Mukherjee J, Phillips JJ, Zheng S, Wiencke J, Ronen SM, Pieper RO (2013) Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS One 8:e57610
Nath S, Roychoudhury S, Kling MJ, Song H, Biswas P, Shukla A, Band H, Joshi S, Bhakat KK (2017) The extracellular role of DNA damage repair protein APE1 in regulation of IL-6 expression. Cell Signal 39:18–31
Redaelli A, Magrassi R, Bonassi S, Abbondandolo A, Frosina G (1998) AP endonuclease activity in humans: development of a simple assay and analysis of ten normal individuals. Teratog Carcinog Mutagen 18:17–26
Sabarinathan D, Mahalakshmi P, Vanisree AJ (2011) Naringenin, a flavanone inhibits the proliferation of cerebrally implanted C6 glioma cells in rats. Chem Biol Interact 189:26–36
Schleicher SM, Thotala DK, Linkous AG, Hu R, Leahy KM, Yazlovitskaya EM, Hallahan DE (2011) Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PLoS One 6:e22182
Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H (2003) Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res 63:1706–1711
Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, Kolstoe DD (2002) The apurinic/apyrimidinic endonuclease activity of Ape1/ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res 8:3008–3018
Singh S, Englander EW (2012) Nuclear depletion of apurinic/apyrimidinic endonuclease 1 (Ape1/ref-1) is an indicator of energy disruption in neurons. Free Radic Biol Med 53:1782–1790
Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, Leonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12:376–390
Tamada, M., Suematsu, M., & Saya, H. (2012). Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells: American Association for Cancer Research
Tell G, Quadrifoglio F, Tiribelli C, Kelley MR (2009) The many functions of APE1/ref-1: not only a DNA repair enzyme. Antioxid Redox Signal 11:601–619
Tell G, Fantini D, Quadrifoglio F (2010) Understanding different functions of mammalian AP endonuclease (APE1) as a promising tool for cancer treatment. Cell Mol Life Sci 67:3589–3608
Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158:227–233
Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-Oncology 4:278–299
Wu J-M, Xu Y, Skill NJ, Sheng H, Zhao Z, Yu M, Saxena R, Maluccio MA (2010) Research Autotaxin expression and its connection with the TNF-alpha-NF-κB axis in human hepatocellular carcinoma. Mol Cancer 9:1–14
Xiangyun Y, Xiaomin N (2017) Desuccinylation of pyruvate kinase M2 by SIRT5 contributes to antioxidant response and tumor growth. Oncotarget 8:6984
Yamada T, Sato K, Komachi M, Malchinkhuu E, Tobo M, Kimura T, Kuwabara A, Yanagita Y, Ikeya T, Tanahashi Y (2004) Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem 279:6595–6605
Yang W, Lu Z (2013) Nuclear PKM2 regulates the Warburg effect. Cell Cycle 12:3343–3347
Acknowledgements
This work is supported to A.K.M. by the BSR-startup grant received from the University Grants Commission (UGC), New Delhi, India, and the funds received under the scheme Research Seed Money (RSM) from the Central University of Punjab, Bathinda (CUPB). R.P.C. acknowledges financial support in the form of a senior research fellowship (SRF) from the Indian Council for Medical Research (ICMR), New Delhi, India. The confocal laser scanning microscope (Olympus) facility of the Central Instrumentation Laboratory (CIL), CUPB is thankfully acknowledged. Because of the limited focus of the article, many relevant and appropriate references could not be included, for which the authors apologize.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Cholia, R.P., Dhiman, M., Kumar, R. et al. Oxidative stress stimulates invasive potential in rat C6 and human U-87 MG glioblastoma cells via activation and cross-talk between PKM2, ENPP2 and APE1 enzymes. Metab Brain Dis 33, 1307–1326 (2018). https://doi.org/10.1007/s11011-018-0233-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0233-3