Abstract
Increasing evidence suggests that the gut microbiota plays a key role in the central nervous system (CNS), and alterations of the gut microbiota composition due to environmental factors can contribute to neurodevelopmental disorders. Animal modeling may help to identify drugs that can normalize the altered gut microbiota and thereby ameliorate abnormal brain signaling pathways. The purpose of the present study was to investigate the therapeutic potency of probiotics such as Bifidobacteria and Lactobacilli on glutamate excitotoxicity as a neurotoxic effect induced by clindamycin and propionic acid (PPA) in juvenile hamsters. Fifty young golden Syrian hamsters weighing between 60 and 70 g were enrolled in the study. The hamsters were randomly divided into five groups, each with ten hamsters. The hamsters in the control group only received phosphate-buffered saline orally. The PPA-treated group received a neurotoxic dose of 250 mg PPA/kg body weight (BW)/day for three days. The clindamycin-treated group received 30 mg clindamycin/kg BW as a single orogastric dose on the day the experiment started. The two therapeutic groups received the same doses of PPA and clindamycin followed by 0.2 g probiotic/kg BW for three weeks. Biochemical parameters related to glutamate excitotoxicity were investigated in brain homogenates from each group of hamsters. Additionally, the development of pathogenic bacteria was monitored in stool samples from all groups. The microbiology results of the present study revealed descriptive changes in the fecal microbiota and the appearance of Clostridium species in the hamsters treated with clindamycin and PPA. Additionally, the effectiveness of the probiotic in the restoration of the normal gut microbiota was demonstrated. Moreover, clindamycin and PPA were found to induce a significant depletion of Mg2+ and γ-aminobutyric acid (GABA) and a remarkable increase in the Na+/Mg2+ and glutamate/GABA ratios but non-significant changes in the absolute levels of K+, Na+ and glutamate. The bacteria overgrowth induced by PPA and clindamycin in the present study effectively showed signs of neuronal toxicity. The study indicates that probiotics can be used safely to ameliorate glutamate excitotoxicity mostly through increasing depleted GABA and Mg2+ and decreasing the excitatory neurotransmitter, glutamate.
Similar content being viewed by others
Introduction
A possible connection between elevated propionic acid (PPA), which is a metabolite of Clostridium difficile, and autism has been suggested and has been utilized to biochemically induce persistent autistic features in rat pups (El-Ansary et al. 2012). Moreover, either oral or intracerebroventricular administration of PPA to rodents induces repetitive behavior, impaired social behavior, seizure activity, and hyperactivity that are similar to autism in humans (MacFabe et al. 2007, 2011; Foley et al. 2014a, b; 2015). The possible suggested mechanisms through which elevated concentrations of PPA affect the brain include oxidative stress, neuroinflammation, impaired energy metabolism, and glutamate excitotoxicity (El-Ansary et al. 2012; Al-Suwailem 2016). In a recent study by Daghestani et al. (2017), exploratory, social, locomotor, and repetitive/stereotype-like activities were found in a rodent model with an orally administered dose of 250 mg PPA/kg body weight (BW)/day for three days. The same dose of PPA was used in the present study.
Many different approaches have been designed to induce autistic features in rodents (MacFabe et al. 2008, 2011). Likewise, clindamycin-treated hamsters are known to be more susceptible to infection with C. difficile and develop many features similar to humans infected with C. difficile. Thus, the development of C. difficile-infected rodent models of autism may be helpful to better understand the role C. difficile in autism. (Michelle et al. 2003). Among the multiple disease mechanisms in autism, the imbalance between excitation and inhibition (E/I) related to glutamate signaling is of critical importance. It is well documented that the E/I balance is important for synaptogenesis and plasticity especially during the early stage of brain development (Oberman 2012). An increased E/I ratio has been thought to be connected with the hyperexcitability associated with ASD development (Rubenstein and Merzenich 2003; Casanova et al. 2009).
A recent study by Kim et al. (2017) noted that a greater E/I imbalance of the medial prefrontal cortex in a rodent model of autism impaired cellular information processing that was specifically related to the social behavior impairment. Moreover, stimulation of inhibitory neurotransmission was found to reverse the impaired social interaction in this model effectively. The importance of the E/I imbalance as a mechanism related to autism was supported by a post-mortem study that demonstrated lower γ-aminobutyric acid (GABA) production secondary to a reduced level of the limiting enzyme glutamic acid decarboxylase (GAD) in PPA-treated rats (Unpublished work), and lower concentrations of GABA will cause imbalance and favor excitability (Fatemi et al. 2009; Gatto and Broadie 2010).
There are recent research interests in the bidirectional crosstalk between the brain and gut microbiota as an important player in the physiology and pathology of mammalian systems (Sampson and Mazmanian 2015). The emerging interest in the role of the gut microbiota in health and diseases has led to investigations of its relationship with certain psychiatric disorders, including autism (Sampson and Mazmanian 2015). The composition of the gut microbiota is well documented to be significantly altered in autistic and stressed patients (de Theije et al. 2014; Li and Zhou 2016; Li et al. 2017; Vuong and Hsiao 2017; Strati et al. 2017). Research has shown that manipulations of the gut microbiota through the administration of high amounts of probiotics as live microorganisms can induce multiple benefits on the host’s health and behavior (Hill et al., 2014). Probiotics may modulate a wide range of pathologic mechanisms related to brain disorders, including oxidative stress, neuroinflammation, and glutamate excitotoxicity (Savignac et al. 2014; Liang et al. 2015; Liu et al. 2016). The effect of probiotics on selected markers related to glutamate signaling has been studied in juvenile hamsters treated with propionic acid and clindamycin, two neurotoxins that induce persistent autistic features in rodent models (El-Ansary et al. 2012). In the present study, sodium (Na), potassium (K), and magnesium (Mg), ions greatly related to neuronal depolarization and excitability, together with glutamate and GABA, excitatory and inhibitory neurotransmitters, respectively, were measured in PPA and clindamycin-treated hamsters in an attempt to understand the role of the gut-microbiota-brain axis in the neurotoxic effect caused by both treatments. Moreover, the present study further tested the role of Bifidobacteria and Lactobacilli in ameliorating the toxic effects of both.
Materials and methods
Animals
The experimental assay of the present study was performed on 50 young male golden Syrian hamsters weighing between 60 and 70 g (7 weeks of age). The hamsters were purchased from a live safari store in Riyadh, Saudi Arabia and were kept individually in a cage. The animals were raised under standard laboratory conditions and fed standard diets (laboratory animal feed pellets obtained from Grain Silos and Flour Mills Organization, Saudi Arabia) and water.
Assay kits from United Diagnostics Industry (Dammam, Saudi Arabia) were used for the analysis of magnesium (REF 050), potassium (REF 051), and sodium (REF 054 K). ELISA kits from MyBioSource (San Diego, USA) were used for the analysis of glutamate (Catalog No MBS087763) and GABA (Catalog No MBS019947). The pharmaceuticals used in the present study were a probiotic (ProtexinR restore, Probiotics International Limited (Somerset, UK)) composed of a mixture of Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, and Lactobacillus bulgaricus in a concentration of 1 billion CFU/sachet or 1 × 109 CFU/day and clindamycin (Dalacin C, Pfizer Middle East, Cairo, Egypt).
Experimental design
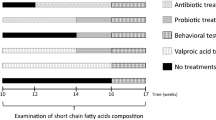
Fifty young male golden Syrian hamsters weighing approximately 60–70 g were maintained in 41 cages (40 × 35 × 20 cm3) at a controlled temperature (21 ± 1 °C) and light conditions (light on at 9:00 and off at 21:00). The hamsters had free access to food (standard laboratory animal feed pellets) and water. The animals were randomly divided into five groups, each of ten animals. During the experimental treatment of the animals, 1, 2, 4, and 2 animals died from the control, clindamycin, PPA, and PPA + probiotic-treated groups, respectively. For the control group, the pups were administered orogastrically doses of PBS daily for 28 days as a vehicle. The hamsters in Group 2 received an orogastric dose of PBS daily till 26 days of a study followed by a single dose of 30 mg clindamycin/kg on the 27th day and were killed next day (El-Ansary et al. 2013). The PPA-only treated group, Group 3, was given 250 mg PPA/kg BW/day through oral gavage for three days beginning on the 24th day of the experiment, and the hamsters were killed at the end of the study. The hamsters in Group 4 received a single dose of 30 mg clindamycin/kg BW orogastrically on the first day of the study followed by the administration of 0.2 g probiotic (ProtexinR) dissolved in PBS /kg BW/day containing a bacterial dose of 2 × 108 CFU from the sources previously mentioned for 27 days. The hamsters in Group 5 received a neurotoxic dose of 250 mg of PPA /kg BW/day PBS buffered orogastrically for three days followed by the administration of 0.2 g probiotic (ProtexinR) dissolved in PBS /kg BW/day until the end of the study. Fecal pellets were collected from all the groups at different intervals according to these treatments using metabolic cages, and sterile tubes (Fig. 1). The collected feces were directly stored at −80 °C until further use. The ethics committee for animal research at King Saud University, Riyadh, approved the experimental protocol of the present study.
Brain homogenate preparation
At the end of the feeding trials, the hamsters were anesthetized with carbon dioxide and decapitated. The brains of the animals were removed from the skulls and dissected into small pieces. One gram of whole brain tissue was homogenized in 10 ml of double distilled water for 2–3 min. The homogenates were then centrifuged for 10 min at 3000 rpm and 4 °C. The obtained supernatants were kept at −80 °C until further analysis.
Biochemical assays
All tests were performed in duplicate, and the average of the two obtained readings was used. Sodium, potassium, and magnesium were measured according to the manufacturer instructions using diagnostic kits from United Diagnostics Industry (Dammam, Saudi Arabia). Glutamate (Catalog No MBS087763) and GABA (Catalog No MBS019947) were measured according to the manufacturer instructions using ELISA kits from MyBioSource (San Diego, USA).
Fecal collection and preparation for microbial analysis
Sterile fecal suspensions from each of the treated groups were dissolved in PBS (0.1 M) at a 1:10 (w/v) ratio (Zhichao et al. 2014). All samples were homogenized using a sonicator for 5 s followed by centrifugation at 5000 rpm for 10 min at −4 °C. Ten-fold serial dilutions of the fecal suspensions were then performed. One milliliter of the supernatant from the original dilution (dilution 0) was added to 9 ml sterile PBS in a tube (dilution 1). The process was repeated until dilution 4 was created, and 0.1 ml of each of the prepared dilutions were loaded and spread on the surface of different culture media. The culture media used included nutrient agar NA (Oxoid, UK), MacConkey agar (Oxoid) for the identification of lactose fermenter Enterobacteriaceae, blood agar (Oxoid), Sabouraud dextrose agar (SDA) for yeast isolation and CCFA (cycloserine-cefoxitin fructose agar) for the isolation and identification of Clostridium difficile. NA blood and MacConkey agar plates were incubated aerobically at 37 °C for 18–24 h, whereas CCFA plates were incubated in an anaerobic jar with 5% CO2 at 37 °C for three days; SDA plates were incubated at 28 °C for 18–24 h.
Bacterial enumeration and identification
Prior to incubation, the bacterial count from the different media was recorded as the colony count per plate. Data were compared between the hamster groups in the study. A higher Clostridium difficile count was obtained following clindamycin treatment. Preliminary bacterial identification was performed by morphological observation on the different media used; Clostridium difficile isolates specifically appeared as fluorescent yellow colonies on CCFA medium. Further identification was made microscopically using the gram staining technique, where single colonies from the various culture media were selected, heated to form a smear, subjected to a Gram staining procedure and then observed under the microscope using an oil immersion lens.
Statistical analysis
The results of the present study were expressed as the means ± S.D. All statistical comparisons between the control group and the PA-, clindamycin- and probiotic-treated hamster groups were performed using SPSS version 16.0. One-way analysis of variance (ANOVA) tests with Dunnett’s test for multiple comparisons was performed. Significance was assigned at the level of P < 0.05. Receiver operating characteristics (ROC) curve analysis was also performed. The area under the curve (AUC), the degrees of sensitivity and specificity, and cutoff values were calculated. Pearson’s correlations were performed between the measured parameters.
Results
The data from the present study are presented in four tables and one figure. Table 1 demonstrates the descriptive changes of the gut microbiota post-PPA and clindamycin treatment together with the therapeutic effect of Bifidobacteria and Lactobacilli. Most noticeable is the induction of the growth of Candida albicans and clostridia species after treatment with PPA and clindamycin and their disappearance upon treatment with the probiotic (ProtexinR). Enterobacteriaceae were only presenting in clindamycin-treated but not in PPA-treated hamsters, and again, the probiotic was effective at stopping its growth.
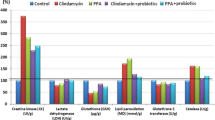
Table 2 and Fig. 2 demonstrate the absolute and relative levels of Na+, K+, Mg2+, glutamate, and GABA in the five studied groups. Fig. 3 represents the percentage change of the measured parameters relative to control. While the concentrations of Na+, K+, and glutamate were non-significantly changed with PPA and clindamycin treatments, there was a significant decrease in Mg2+ with both treatments (68.66 and 70.15%, respectively), and the Na+/Mg2+ ratio was significantly altered with both treatments but back to normal after treatment with the probiotic. Moreover, there was a significant decrease in GABA with PPA treatment (22.14%).
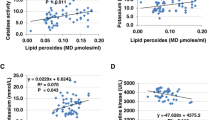
Table 3 demonstrates the positive and negative correlations between the measured parameters. Notably, while Na+ and K+ were positively correlated with the absolute and relative concentrations of both glutamate and GABA, Mg2+ only positively correlated with the concentration of GABA.
Table 4 shows the ROC analysis of the measured parameters. Clearly, Mg2+ and Na+/Mg2+, as well as GABA, had a high AUC together with high sensitivity and specificity for the two intoxicated groups (PPA- and clindamycin-treated hamsters). Moreover, GABA and glutamate/GABA had a high AUC and high specificity and sensitivity in both cases (in cases of neurotoxicity and those showing the therapeutic effects of the probiotic).
Discussion
Neuronal excitability and excessive depolarization are usually associated with a significant alteration in extracellular ion concentrations. These changes are mostly due to transmembrane ion flux through transporters and ion channels that contribute to these processes (Heinemann et al. 2017). Ion channels are well known to be important regulators of intrinsic excitability. A fragile X model of mentally retarded mice showed reduced expression together with the impaired function of dendritic cell big potassium channels (BKCa) in cortical neurons associated with hyperexcitability and hypersensitivity, which were both corrected through BKCa stimulation (Zhang et al. 2014). Moreover, neuronal excitability is usually increased by the inhibition of voltage-dependent potassium channels, leading to loss of the regulatory signaling of the interneuronal GABAergic output (Li et al. 2011; Tan et al. 2011). Voltage-gated sodium channels are related to the impairment and suppression of GABAergic signaling, characterized by seizures together with cognitive and social deficits (Han et al. 2012; Ito et al. 2013). Based on the fact that magnesium has been shown to block N-methyl-D-aspartate (NMDA) receptors as an important component of glutamate neurotransmission in the brain, decreased concentrations of neuronal magnesium are believed to be related to brain excitability (Dube and Granry 2003). While a high concentration of Mg2+ has a depressive effect on NMDA-responses and thus can regulate the excitatory response in general, a lower extracellular Mg2+ concentration below the physiological level can remarkably potentiate NMDA receptors and thus induce glutamate excitotoxicity (Ault et al. 1980).
Table 1 and Fig. 1 present the relative and absolute levels of Na+, K+, and Mg2+. Although both Na+ and K+ showed non-significant changes between PPA-, clindamycin-, and clindamycin–probiotic-treated animals, there was a highly significant decrease in Na+ in PPA-probiotic-treated hamsters compared to that in healthy controls.
Because many brain enzymes require Mg2+, the remarkable lower level of Mg2+ in both the PPA- and the clindamycin-treated groups may be related to the significant toxic effects reported in the present study. The intracellular effects of Mg2+ ions mainly oppose those of Ca2+ ions, and Mg2+ deficiency-induced brain seizures can be avoided by NMDA-receptor antagonists (Krnjevic et al. 1979; Morris 1992; Durlach and Bac 1997). Therefore, the significant reduction in Mg2+ shown in the present study as a marker of PPA- and clindamycin neurotoxicity may be associated with glutamate excitotoxicity. These observations indicate that glutamate excitotoxicity may be an etiological factor in PA-induced persistent autistic features in juvenile rats (El-Ansary 2016; El-Ansary et al. 2017). In the case of Mg2+ deficiency, excessive Ca2+ and glutamate can easily induce brain cell synaptic dysfunction, which can be observed as repetitive behavior, impaired social behavior, seizure activity, and hyperactivity as previously reported by MacFabe et al. (2007, 2011), Al-Suwailem (2016), and Daghestani et al. (2017). Moreover, the neurotoxic effect of Mg2+ deficiency in the present study may find support in the earlier work of MacFabe et al. (2011) and El-Ansary et al. (2012, 2013), which reported abnormal serotonin, dopamine, noradrenaline, glutamate, and Na+/K+-ATPase activity among the biochemically induced persistent autistic features in rat pups and/or hamsters through the use of PPA and clindamycin (Durlach and Bac 1997; Brudnak 2002; El-Ansary et al. 2012). In the present study, the non-significant alteration in the Na+/K+ ratio (Fig. 1 and Table 1), together with the significant depletion of Mg2+, may be enough to induce Na+/K+-ATPase activity as a mechanism of neurotoxicity caused by Mg2+ depletion (Durlach and Bac 1997).
The present data demonstrate the beneficial effect of the tested probiotic (ProtexinR), which is a mixture of Bifidobacteria and Lactobacilli strains. While clindamycin treatment did not induce the growth of Candida, probiotic supplementation has been found to be effective in reducing the growth of Candida (Brudnak 2002). This finding is in agreement with Payne et al. who found that Candida growth was reduced when a probiotic species, Lactobacillus plantarum, was added to the medium (Payne et al. 2003). Moreover, the beneficial effects of Bifidobacteria and Lactobacilli in ameliorating the symptoms of autism have repeatedly been observed in studies and clinical trials (Douglas and Sanders 2008; Parracho et al. 2010).
Blaylock (1999) reported that extensive consumption of food additive excitotoxins such as glutamate, aspartate, and PPA is strongly correlated to endogenous excitotoxicity as an important mental illness phenotype. The therapeutic effect of the probiotic supplement used in the present study is presented as the restoration of the normal brain Mg+2 levels with a concomitant decrease in glutamate and increase in GABA in PPA-treated hamsters. This result is supported by the previous work of Stein and Glasier (1992) in which they showed that glutamate excitotoxicity could be treated with Mg+2. These observations suggest that alterations in gut biota are related to glutamate excitotoxicity and Mg+2 depletion (Filpa et al. 2016). A depletion of Mg2+ that blocks NMDA receptors usually exacerbates the neurotoxic effects of glutamate and thereby increases the neurotoxic effects of PPA and clindamycin (Yasui et al. 1997).
Table 3 demonstrates the positive and negative correlations between the measured parameters. The positive relationship between Na+, Na+/K+, glutamate, and glutamate/GABA demonstrate the role of sodium in neuronal depolarization and excitability. This role is supported by the observed decrease in the Na+ level after probiotic treatment. The previously explained role of Mg+2 depletion in glutamate excitotoxicity was also demonstrated by a positive correlation between Mg+2 and GABA. The significant elevation in Mg+2 by the probiotic was accompanied by a remarkable increase in the inhibitory neurotransmitter GABA, which may help to restore the excitatory/inhibitory balance.
Table 4 shows the ROC analysis of the measured parameters. The comparison of the AUC of the different markers in the four studied groups suggests that Mg2+, Na+/Mg2+, and GABA may be predictive markers of PPA and clindamycin neurotoxicity. On the other hand, GABA and glutamate/GABA demonstrate acceptable validity as measures of PPA neurotoxicity together with the therapeutic potency of the tested probiotic, ProtexinR.
Conclusion
Overall, the findings of the present study indicate that bacteria overgrowth induced by PPA and clindamycin effectively induces signs of neuronal toxicity. Additionally, Bifidobacteria and Lactobacilli supplementation can be suggested as a strategy to ameliorate the glutamate excitotoxic effects of PPA and clindamycin.
References
Al-Suwailem EA (2016) Understanding glutamate signaling defects in propionic acid-orally administered wistar rat pups as animal model of autism. Biochemistry Department, College of Science, King Saud University, Riyadh, Master thesis
Ault B, Evans RH, Francis AA, Oakes DJ, Watkins JC (1980) Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol 307:413–428
Blaylock R (1999) Food additive excitotoxins and degenerative brain disorders. Med Sentinel 4:212–215
Brudnak MA (2002) Probiotics as an adjuvant to detoxification protocols. Med Hypotheses 58:382–395
Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C (2009) Minicolumnar abnormalities in autism. Acta Neuropathol 112:287–303
Daghestani MH, Selim ME, Abd-Elhakim YM, Said EN, Abd El-Hameed NE, Khalil SR, El-Tawil OS (2017) The role of apitoxin in alleviating propionic acid-induced neurobehavioral impairments in rat pups: the expression pattern of Reelin gene. Biomed Pharmacother 93:48–56
de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD, Oozeer R (2014) Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37:197–206
Douglas LC, Sanders ME (2008) Probiotics and prebiotics in dietetics practice. J Am Diet Assoc 108:510–521
Dube L, Granry JC (2003) The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: a review. Can J Anaesth 50:732–746
Durlach J, Bac P (1997) Mechanisms of action on the nervous system in magnesium deficiency and dementia. In: Yasui M, Strong MJ, Ota K, Verity MA (eds) Mineral and metal neurotoxicology. CRC press, Boca Raton, pp 201–209
El-Ansary (2016) A data of multiple regressions analysis between selected biomarkers related to glutamate excitotoxicity and oxidative stress in Saudi autistic patients. Data Brief 7:111–116
El-Ansary AK, Ben Bacha A, Kotb M (2012) Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation 9(74). https://doi.org/10.1186/1742-2094-9-74
El-Ansary A, Shaker G, Siddiqi NJ, Al-Ayadhi LY (2013) Possible ameliorative effects of antioxidants on propionic acid/clindamycin - induced neurotoxicity in Syrian hamsters. Gut Pathog 5:32. https://doi.org/10.1186/1757-4749-5-32
El-Ansary A, Al-Salem HS, Asma A, Al-Dbass A (2017) Glutamate excitotoxicity induced by orally administered propionic acid, a short chain fatty acid can be ameliorated by bee pollen. Lipids Health Dis 16(96):96. https://doi.org/10.1186/s12944-017-0485-7
Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD (2009) GABA(a) receptor downregulation in brains of subjects with autism. J Autism Dev Disord 39:223–230
Filpa V, Moro E, Protasoni M, Crema F, Frigo G, Giaroni C (2016) Role of glutamatergic neurotransmission in the enteric nervous system and brain-gut axis in health and disease. Neuropharmacology 111:14–33
Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014a; 9(1):e87072. https://doi.org/10.1371/journal.pone.0087072. eCollection 2014a
Foley KA, MacFabe DF, Kavaliers M, Ossenkopp KP (2015) Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: relevance to autism spectrum disorders. Behav Brain Res 278:244–256. https://doi.org/10.1016/j.bbr.2014.09.032
Foley KA, MacFabe DF, Vaz A, Ossenkopp KP, Kavaliers M (2014b) Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int J Dev Neurosci 39:68–78. https://doi.org/10.1016/j.ijdevneu.2014.04.001
Gatto CL, Broadie K (2010) Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci 7:2–4
Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA (2012) Autistic-like behaviour in Scn1a1/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489:385–390
Heinemann U, Angamo EA, Liotta A (2017) Non-synaptic mechanisms: modulation of neuronal excitability by changes in extracellular ion composition. Reference Module in Neuroscience and Biobehavioral Psychology. https://doi.org/10.1016/B978-0-12-809324-5.00163-2
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document: the international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Ito S, Ogiwara I, Yamada K, Miyamoto H, Hensch TK, Osawa M, Yamakawa K (2013) Mouse with Nav1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis 49:29–40
Kim JW, Seung H, Kim KC, Gonzales EL, Oh HA, Yang SM, Ko MJ, Han SH, Banerjee S, Shin CY (2017) Agmatine rescues autistic behaviors in the valproic acid-induced animal model of autism. Neuropharmacology 113(Pt A):71–81
Krnjevic K, Lamour Y, MacDonald JF, Nistri A, Puil E, Werman R (1979) Intracellular divalent cations and neuronal excitability. Can J Physiol Pharmacol 57:957–972
Li Q, Zhou JM (2016) The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 324:131–139
Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM, Cao SX, Chen XJ, Chen Z, Luo JH, Duan S, Li XM (2011) Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci 15:267–273
Li Q, Han Y, Dy ABC, Hagerman RJ (2017) The gut microbiota and autism spectrum disorders. Front Cell Neurosci 11(120). https://doi.org/10.3389/fncel.2017.00120
Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F (2015) Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310:561–577
Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, Wang S, Tsai YC (2016) Psychotropic effects of lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res:1631:1–163112
MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, Taylor AR, Kavaliers M, Ossenkopp KP (2007) Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res 176:149–169
MacFabe DF, Rodríguez-Capote K, Hoffman JE, Franklin AE, Mohammad-Asef Y, Taylor AR, Boon F, Cain DP, Kavaliers M, Possmayer F, Ossenkopp KP (2008) Novel rodent model of autism: Intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotechnol 4:146–166. https://doi.org/10.3844/ajbbsp.2008.146.166
MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP (2011) Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res 217:47–54
Michelle M, Susan S, Stuart J, Dale N (2003) Susceptibility of hamsters to human pathogenic clostridium difficile strainB1 following clindamycin, ampicillin or ceftriaxone administration. Anaerobe 9:91–95
Morris ME (1992) Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes Res 5:303–313
Oberman LM (2012) mGluR antagonists and GABA agonists as novel pharmacological agents for the treatment of autism spectrum disorders. Expert Opin Investig Drugs 21:1819–1825
Parracho HMRT, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL (2010) A double-blind, placebo controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int J Probiotics Prebiotics 5:69–74
Payne S, Gibson G, Wynne A, Hudspith B, Brostoff J, Tuohy K (2003) In vitro studies on colonization resistance of the human gut microbiota to Candida albicans and the effects of tetracycline and lactobacillus plantarum LPK. Curr Issues Intest Microbiol 4:1–8
Rubenstein JL, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2:255–267
Sampson TR, Mazmanian SK (2015) Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17:565–576
Savignac HM, Kiely B, Dinan TG, Cryan JF (2014) Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil 26:1615–1627
Stein DG, Glasier MM (1992) An overview of developments in research on recovery from brain injury. In: Rose FD, Johnson DA (eds) Recovery from brain damage reflections and directions. Plenum press, New York, pp 1–22
Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, De Filippo C (2017) New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5(1):24
Tan GH, Liu YY, Hu XL, Yin DM, Mei L, Xiong ZQ (2011) Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbuminexpressing interneurons. Nat Neurosci 15:258–266
Vuong HE, Hsiao EY (2017) Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry 81:411–423
Yasui M, Ota K, Murphy VA (1997) Magnesium-related neurological disorders. In: Yasui M, Strong MJ, Ota K, Verity MA (eds) Mineral and metal neurotoxicology. CRC press, Boca Raton, pp 219–226
Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, Frick A (2014) Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(−/y) mice. Nat Neurosci 17:1701–1709
Zhichao Z, Xichun P, Saoting L, Ning Z, Yong W, Hua W (2014) Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS One 9:e90531. https://doi.org/10.1371/journal.pone.0090531
Acknowledgements
This research project was supported by the Deanship of Scientific Research, Princess Nora Bint Abdulrahman University, Grant number 38-213. Thanks also are due to Ms. Shaista Arzoo, for her appreciated effort in revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
El-Ansary, A., Bacha, A.B., Bjørklund, G. et al. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab Brain Dis 33, 1155–1164 (2018). https://doi.org/10.1007/s11011-018-0212-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0212-8