Abstract
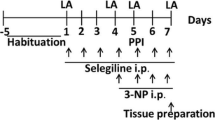
The aim of the present study was to investigate the effects of SCH58261, a selective adenosine A2A receptor antagonist, on striatal toxicity induced by 3-nitropropionic acid (3-NP) in rats. The experimental protocol consisted of 10 administrations (once a day) of SCH58261 (0.01 or 0.05 mg/kg/day, intraperitoneal, i.p.). From 7th to 10th day, 3-NP (20 mg/kg/day, i.p.) was injected 1 h after SCH58261 administration. Twenty-four hours after the last 3-NP injection, the body weight gain, locomotor activity (open-field test), motor coordination (rotarod test), striatal succinate dehydrogenase (SDH) activity and parameters linked to striatal oxidative status were evaluated in rats. The marked body weight loss resulting from 3-NP injections in rats was partially protected by SCH 58261 at both doses. SCH 58261 at the highest dose was effective against impairments on motor coordination and locomotor activity induced by 3-NP. SCH 58261 was unable to restore the inhibition of SDH activity caused by 3-NP. In addition, the increase in striatal reactive species (RS) levels, depletion of reduced glutathione (GSH) content and stimulation of glutathione reductase (GR) activity provoked by 3-NP injections were alleviated by both doses of SCH 58261. The highest dose of SCH 58261 was also effective in attenuating the increase of protein carbonyl levels as well as the inhibition of glutathione peroxidase (GPx) activity in rats exposed to 3-NP. Our results revealed that reduction of oxidative stress in rat striatum by adenosine A2A receptor antagonism contributes for alleviating 3-NP-induced toxicity.




Similar content being viewed by others
References
Allison C, Pratt JA (2003) Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol Therapeut 98:171–195
Backos DS, Franklin CC, Reigan P (2012) The role of glutathione in brain tumor drug resistance. Biochem Pharmacol 83:1005–1012
Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, Saudou F, Elalouf JM, Hirsch E, Hantraye P, Deglon N, Brouillet E (2006) Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell 17:1652–1663
Blum D, Galas MC, Pintor A, Brouillet E, Ledent C, Muller CE, Bantubungi K, Galluzzo M, Gall D, Cuvelier L, Rolland AS, Popoli P, Schiffmann SN (2003) A dual role of adenosine A(2A) receptors in 3-nitropropionic acid-induced striatal lesions: implications for the neuroprotective potential of A(2)A antagonists. J Neurosci 23:5361–5369
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Browne SE, Beal MF (2004) The energetics of Huntington’s disease. Neurochem Res 29:531–546
Browne SE, Ferrante RJ, Beal MF (1999) Oxidative stress in Huntington’s disease. Brain Pathol 9:147–163
Canas PM, Porciuncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA (2009) Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci 29:14741–14751
Carlberg I, Mannervik B (1985) Glutathione-reductase. Methods Enzymol 113:484–490
Chaturvedi RK, Beal MF (2013) Mitochondria targeted therapeutic approaches in Parkinson’s and Huntington's diseases. Mol Cell Neurosci 55:101–114
Chen NS, Luo T, Wellington C, Metzler M, McCutcheon K, Hayden MR, Raymond LA (1999) Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J Neurochem 72:1890–1898
Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT (2007) Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochem Biophys Res Commun 359:335–340
Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38:107–125
Domenici MR, Scattoni ML, Martire A, Lastoria G, Potenza RL, Borioni A, Venerosi A, Calamandrei G, Popoli P (2007) Behavioral and electrophysiological effects of the adenosine A2A receptor antagonist SCH 58261 in R6/2 Huntington's disease mice. Neurobiol Dis 28:197–205
Fink JS, Kalda A, Ryu H, Stack EC, Schwarzschild MA, Chen JF, Ferrante RJ (2004) Genetic and pharmacological inactivation of the adenosine A2A receptor attenuates 3-nitropropionic acid-induced striatal damage. J Neurochem 88:538–544
Fu JR, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, Peng Q, Jiang M, Arbez N, Hotaling K, Ross CA, Duan WZ (2012) Trans-(-)-epsilon-viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of huntington disease. J Biol Chem 287:24460–24472
Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA (2011) Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808:1380–1399
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Huang NK, Lin JH, Lin JT, Lin CI, Liu EM, Lin CJ, Chen WP, Shen YC, Chen HM, Chen JB, Lai HL, Yang CW, Chiang MC, Wu YS, Chang C, Chen JF, Fang JM, Lin YL, Chern Y (2011) A new drug design targeting the adenosinergic system for Huntington's disease. PLoS One 6:e20934
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4:1399–1440
Klepac N, Relja M, Klepac R, Hecimovic S, Babic T, Trkulja V (2007) Oxidative stress parameters in plasma of Huntington's disease patients, asymptomatic Huntington's disease gene carriers and healthy subjects : a cross-sectional study. J Neurol 254:1676–1683
Kumar P, Padi SS, Naidu PS, Kumar A (2007) Cyclooxygenase inhibition attenuates 3-nitropropionic acid-induced neurotoxicity in rats: possible antioxidant mechanisms. Fundam Clin Pharmacol 21:297–306
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin J-P, Mankhetkorn S (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat Phys Chem 72:323–331
Long JD, Matson WR, Juhl AR, Leavitt BR, Paulsen JS (2012) 8OHdG as a marker for Huntington disease progression. Neurobiol Dis 46:625–634
Maucksch C, Vazey EM, Gordon RJ, Connor B (2013) Stem cell-based therapy for Huntington's disease. J Cell Biochem 114:754–763
Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E (1998) Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport 9:3955–3959
Park JH, Lee HJ, Koh SB, Ban JY, Seong YH (2004) Protection of NMDA-induced neuronal cell damage by methanol extract of Zizyphi Spinosi Semen in cultured rat cerebellar granule cells. J Ethnopharmacol 95:39–45
Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pezzola A, Scarchilli L, Quarta D, Reggio R, Malchiodi-Albedi F, Falchi M, Massotti M (2002) Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci 22:1967–1975
Ramaswamy S, McBride JL, Herzog CD, Brandon E, Gasmi M, Bartus RT, Kordower JH (2007) Neurturin gene therapy improves motor function and prevents death of striatal neurons in a 3-nitropropionic acid rat model of Huntington's disease. Neurobiol Dis 26:375–384
Santos AR, De Campos RO, Miguel OG, Cechinel-Filho V, Yunes RA, Calixto JB (1999) The involvement of K+ channels and Gi/o protein in the antinociceptive action of the gallic acid ethyl ester. Eur J Pharmacol 379:7–17
Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros JQ, Cabiscol E (2008) Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radical Bio Med 45:667–678
Tunez I, Montilla P, Munoz MC, Drucker-Colin R (2004) Effect of nicotine on 3-nitropropionic acid-induced oxidative stress in synaptosomes. Eur J Pharmacol 504:169–175
Tunez I, Tasset I, Perez-De La Cruz V, Santamaria A (2010) 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: past, present and future. Molecules 15:878–916
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Wu JH, Batist G (2013) Glutathione and glutathione analogues: therapeutic potentials. Biochim Biophys Acta 1830:3350–3353
Acknowledgements
We gratefully acknowledge UFPel, UNIFRA, FAPERGS (research grants #16/2551-0000526-5 – PRONUPEQ, # 16/2551-0000240-1 – PRONEM), CAPES and CNPq for the financial support. C.R.J. and C.L. are recipients of CNPq fellowship. M.P.P. is recipients of CAPES fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Bortolatto, C.F., Reis, A.S., Pinz, M.P. et al. Selective A2A receptor antagonist SCH 58261 modulates striatal oxidative stress and alleviates toxicity induced by 3-Nitropropionic acid in male Wistar rats. Metab Brain Dis 32, 1919–1927 (2017). https://doi.org/10.1007/s11011-017-0086-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0086-1