Abstract
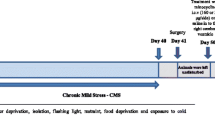
Agomelatine (AGOM) as an antidepressant acts both as a melatonin-receptor agonist and a selective serotonin-receptor antagonist. As a potent melatonin derived antioxidant, AGOM might modulate depression-induced lipid peroxidation and pro-inflammatory cytokines in brain, kidney and liver. The present study explores whether AGOM protects against experimental depression-induced brain, kidney and liver oxidative stress, and plasma cytokine production in rats with chronic mild stress (CMS)-induced depression. Thirty-six rats were divided into four groups. The first group was used as an untreated control. The second group received AGOM for 4 weeks. The third group was exposed to chronic mild stress (CMS) of 4 weeks for induction depression. The fourth group received 40 mg/kg AGOM and CMS for 4 weeks. Liver and kidney lipid peroxidation levels were high in the CMS group although they were low in AGOM treatments. AGOM and AGOM + CMS treatments increased the lowered glutathione peroxidase activity and reduced glutathione levels in brain, kidney and liver of CMS group. β-carotene, vitamin A and vitamin E concentrations in the brain, kidney and liver of the four groups were not changed by CMS and AGOM treatments. However, plasma TNF-α, interleukin (IL)-1β, and IL-4 levels were high in the CMS and AGOM group and their levels were further increased by the AGOM + CMS treatment. In conclusions, AGOM induced protective effects against experimental depression-induced brain, kidney, and liver oxidative injuries through regulation of the glutathione concentrations and glutathione peroxidase activity. However, plasma cytokine productions were increased by the AGOM treatment.



Similar content being viewed by others

Abbreviations
- AGOM:
-
agomelatine
- CMS:
-
chronic mild stress
- GSH:
-
reduced glutathione
- GSH-Px:
-
glutathione peroxidase
- ROS:
-
reactive oxygen species
References
Akpinar A, Uğuz AC, Nazıroğlu M (2014) Agomelatine and duloxetine synergistically modulates apoptotic pathway by inhibiting oxidative stress triggered intracellular calcium entry in neuronal PC12 cells: role of TRPM2 and voltage-gated calcium channels. J Membr Biol 247:451–459
Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M (2007) A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun 21:147–152
Aygün H, Aydın D, İnanır S, Ekici F, Ayyıldız M, Ağar E (2015) The effects of agomelatine and melatonin on ECoG activity of absence epilepsy model in WAG/Rij rats. Turk J Biol 39:904–910
Bakunina N, Pariante CM, Zunszain PA (2015) Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144:365–373
Balaban H, Nazıroğlu M, Demirci K (2016) The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: The involvement of TRPM2 and TRPV1 channels. doi:10.1007/s12035-016-9835-0
Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA (2007) Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun 21:229–237
Dagyte D, Crescente I, Postema F, Seguin L, Gabriel C, Mocaer E, Den Boer JA, Jaap Koolhaas JM (2011) Agomelatine reverses the decrease in hippocampal cell survival induced by chronic mild stress. Behav Brain Res 218:121–128
de Mello AH, da Rosa SL, Moreira Cereja AC, de Bona SR, Florentino D, Modolon Martins M, Petronilho F, Quevedo J, Tezza Rezin G (2015) Effect of subchronic administration of agomelatine on brain energy metabolism and oxidative stress parameters in rats. Psychiatry Clin Neurosci. doi:10.1111/pcn.12371
Demyttenaere K (2011) Agomelatine: a narrative review. Eur Neuropsychopharmacol 21: S703–S709.
Desai ID (1984) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–147
Ekmekcioglu C. (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 60:97–108.
Eren I, Nazıroğlu M, Demirdaş A (2007a) Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res 32:1188–1195
Eren I, Naziroğlu M, Demirdaş A, Celik O, Uğuz AC, Altunbaşak A, Ozmen I, Uz E (2007b) Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem Res 32:497–505
Espino J, Bejarano I, Paredes SD, Barriga C, Rodríguez AB, Pariente JA (2011) Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J Pineal Res 51:195–206
Freiesleben SD, Furczyk K (2015) A systematic review of agomelatine-induced liver injury. J Mol Psych 3:4
Gupta S, Sharma B (2014) Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol Biochem Behav 122:122–135
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Inanir S, Copoglu US, Kokacya H, Dokuyucu R, Erbas O, Inanır A (2015) Agomelatine protection in an LPS-induced psychosis-relevant behavior model. Med Sci Monit 21:3834–3839
Kahya MC, Naziroğlu M, Çiğ B. (2015) Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain Inj 29:1490–1406.
Karakus E, Halici Z, Albayrak A, Polat B, Bayir Y, Kiki I, Cadirci E, Topcu A, Aksak S (2013) Agomelatine: An antidepressant with new potent hepatoprotective effects on paracetamol-induced liver damage in rats. Hum Exp Toxicol 32:846–857
Kharwar RK, Haldar C (2012) Daily variation in antioxidant enzymes and lipid peroxidation in lungs of a tropical bird Perdicula asiatica: role of melatonin and nuclear receptor RORα. Comp Biochem Physiol A Mol Integr Physiol 162:296–302
Kumar H, Sharma BM, Sharma B (2015) Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochem Int 91:34–45
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin- Phenol reagent. J Biol Chem 193:265–275
Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D (2003) The novel melatonin agonist Agomelatine (S20098) Is anantagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306:954–964
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Nazıroğlu M, Demirdaş A (2015) Psychiatric disorders and TRP channels: Focus on psychotropic drugs. Curr Neuropharmacol 13:248–257
Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. (2002) Preclinical models: status of basic research in depression. Biol Psychiatry 52:503–528
Nicholson TE, Renton KW (2001) Role of cytokines in the lipopolysaccharide-evoked depression of cytochrome P450 in the brain and liver. Biochem Pharmacol 62:1709–1717
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann’ s reagent. Anal Biochem 25:192–205
Senol N, Nazıroğlu M, Yürüker V (2014) N-acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem Res 39:685–692
Shirazi A, Mihandoost E, Ghobadi G, Mohseni M, Ghazi-Khansari M (2013) Evaluation of radioprotective effect of melatonin onwhole body irradiation induced liver tissue damage. Cell J 14:292–297
Sierra-Honigmann MR, Murphy PA (1992) Suppression of interleukin–1 action by phospholipase-A2 inhibitors in helper T lymphocytes. Pept Res 5:258–261
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Tardito D, Molteni R, Popoli M, Racagni G (2012) Synergisticmechanisms involved in the antidepressant effects of agomelatine. Eur Neuropsychopharmacol 22:S482–S486
Vaváková M, Ďuračková Z, Trebatická J (2015) Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Med Cell Longev 12:898393
Voican CS, Corruble E, Naveau S, Perlemuter G (2014) Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry 171:404–415
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–329.
Acknowledgments
The abstract of the study was submitted to the 6th World Congress of Oxidative Stress, Calcium Signaling and TRP Channels, held 24 and 27 May 2016 in Isparta, Turkey (www.cmos.org.tr). The authors wish to thank researcher Bilal Çiğ and technician Muhammet Şahin (Neuroscience Research Center, SDU, Isparta, Turkey) for helping with the cytokine, lipid peroxidation and antioxidant analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
AD and MN formulated the hypothesis and was responsible for writing the report. GÖÜ was responsible for the animal experiments.
Compliance with ethical standards
ᅟ
Financial disclosure
There is no financial disclosure for the current study.
Conflict of interest
None of the authors have any conflicts to disclose. All authors approved the final manuscript.
Rights and permissions
About this article
Cite this article
Demirdaş, A., Nazıroğlu, M. & Ünal, G.Ö. Agomelatine reduces brain, kidney and liver oxidative stress but increases plasma cytokine production in the rats with chronic mild stress-induced depression. Metab Brain Dis 31, 1445–1453 (2016). https://doi.org/10.1007/s11011-016-9874-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9874-2