Abstract
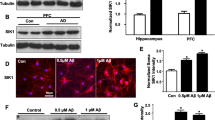
There is sufficient evidence that diabetes during pregnancy is associated with a higher risk of neurodevelopmental anomalies including learning deficits, behavioral problems and motor dysfunctions in the offspring. Synaptophysin (SYP) is an integral membrane protein of synaptic vesicles and is considered as a marker for synaptogenesis and synaptic density. This study aimed to examine the effects of maternal diabetes in pregnancy on the expression and localization of SYP in the developing rat cerebellum. Wistar female rats were maintained diabetic from a week before pregnancy through parturition and male offspring was euthanized at postnatal day (P) 0, 7, and 14. The results revealed a significant down-regulation in the mRNA expression of SYP in the offspring born to diabetic animals at both P7 and P14 (P < 0.05 each). One week after birth, there was a significant reduction in the localization of SYP expression in the external granular (EGL) and in the molecular (ML) layers of neonates born to diabetic animals (P < 0.05 each). We also found a marked decrease in the expression of SYP in all of the cerebellar cortical layers of STZ-D group pups at P14 (P < 0.05 each). Moreover, our results revealed no significant changes in either expression or localization of SYP in insulin-treated group pups when compared with the controls (P ≥ 0.05 each). The present study demonstrated that maternal diabetes has adverse effects on the synaptogenesis in the offspring’s cerebellum. Furthermore, the rigid maternal blood glucose control in the most cases normalized these negative impacts.


Similar content being viewed by others
References
Aerts L, Holemans K, Van Assche FA (1990) Maternal diabetes during pregnancy: consequences for the offspring Diabetes/metabolism reviews 6:147–167
Alladi P, Wadhwa S, Singh N (2002) Effect of prenatal auditory enrichment on developmental expression of synaptophysin and syntaxin 1 in chick brainstem auditory nuclei. Neuroscience 114:577–590
Allen V et al. (2007) Teratogenicity associated with pre-existing and gestational diabetes Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada:. JOGC 29:927–944
Altman J (1972) Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol 145:353–397. doi:10.1002/cne.901450305
Altman J, Bayer SA (1996) Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Functions. CRC Press,
Altman J, Winfree AT (1977) Postnatal development of the cerebellar cortex in the rat. V. Spatial organization of purkinje cell perikarya. J Comp Neurol 171:1–16. doi:10.1002/cne.901710102
Anderson JL, Waller DK, Canfield MA, Shaw GM, Watkins ML, Werler MM (2005) Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology 16:87–92
Arthur CP, Stowell MH (2007) Structure of synaptophysin: a hexameric MARVEL-domain channel protein. Structure 15:707–714
Babiker O (2007) Long-term effects of maternal diabetes on their offspring development and behaviours Sudanese. J Pediatr 8:133–146
D’Agostino AN, Bahn RC (1963) A histopathologic study of the pancreas of infants of diabetic mothers. Diabetes 12:327–331
Baydas G, Nedzvetskii VS, Nerush PA, Kirichenko SV, Yoldas T (2003) Altered expression of NCAM in hippocampus and cortex may underlie memory and learning deficits in rats with streptozotocin-induced diabetes mellitus. Life Sci 73:1907–1916
Bhattacharya B, Sarkar P (1991) Tubulin gene expression during synaptogenesis in rat, mouse and chick brain. Int J Dev Neurosci 9:89–99
Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT (1990) Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci 87:5568–5572
Butts T, Green MJ, Wingate RJ (2014) Development of the cerebellum: simple steps to make a ‘little brain’ Development 141:4031–4041
Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR (1996) Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol 25:821–828
Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R (2002) Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 59:449–456
Cardell B (1953) Hypertrophy and hyperplasia of the pancreatic islets in new-born infants. J Pathol Bacteriol 66:335–346
Cederberg J, Picard JJ, Eriksson UJ (2003) Maternal diabetes in the rat impairs the formation of neural-crest derived cranial nerve ganglia in the offspring. Diabetologia 46:1245–1251. doi:10.1007/s00125-003-1100-1
Chambers JS, Thomas D, Saland L, Neve RL, Perrone-Bizzozero NI (2005) Growth-associated protein 43 (GAP-43) and synaptophysin alterations in the dentate gyrus of patients with schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 29:283–290
Chen G, Wu X, Tuncdemir S (2007) Cell adhesion and synaptogenesis Sheng li xue bao:[Acta physiologica Sinica] 59:697–706
Chua JJ, Kindler S, Boyken J, Jahn R (2010) The architecture of an excitatory synapse. J Cell Sci 123:819–823
Davis EK (2008) Regulation of hippocampal synapse formation and specificity
Davletov B, Montecucco C (2010) Lipid function at synapses. Curr Opin Neurobiol 20:543–549
De Zeeuw CI, Strata P, Voogd J (1997) The Cerebellum: From Structure to Control. Elsevier,
Delascio Lopes C, Sinigaglia-Coimbra R, Mazzola J, Camano L, Mattar R (2011) Neurofunctional evaluation of young male offspring of rat dams with diabetes induced by streptozotocin. ISRN Endocrinology 2011
Diamond A (2000) Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev 71:44–56
Dityatev A, El-Husseini A (2006) Molecular mechanisms of synaptogenesis. Springer Science & Business Media,
Eastwood S, Burnet P, McDonald B, Clinton J, Harrison P (1994) Synaptophysin gene expression in human brain: a quantitative in situ hybridization and immunocytochemical study. Neuroscience 59:881–892
Eastwood S, Burnet P, Harrison P (1995) Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience 66:309–319
Eccles JC (2013) The cerebellum as a neuronal machine. Springer Science & Business Media,
Edelmann L, Hanson P, Chapman E, Jahn R (1995) Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J 14:224
Eidelman AI, Samueloff A (2002) The pathophysiology of the fetus of the diabetic mother. In: Semin Perinatol, vol 3. Elsevier, pp 232–236
Elferink LA, Scheller RH (1993) Synaptic vesicle proteins and regulated exocytosis. J Cell Sci 1993:75–79
Elibol-Can B, Kilic E, Yuruker S, Jakubowska-Dogru E (2014) Investigation into the effects of prenatal alcohol exposure on postnatal spine development and expression of synaptophysin and PSD95 in rat hippocampus. Int J Dev Neurosci 33:106–114
Eriksson UJ (2009) Congenital anomalies in diabetic pregnancy. In: Semin Fetal Neonatal Med, 2009. vol 2. Elsevier, pp 85–93
Eriksson UJ, Simán CM (1996) Pregnant diabetic rats fed the antioxidant butylated hydroxytoluene show decreased occurrence of malformations in offspring. Diabetes 45:1497–1502
Eriksson UJ, Bone AJ, Turnbull DM, Baird JD (1989a) Timed interruption of insulin therapy in diabetic BB/E rat pregnancy: effect on maternal metabolism and fetal outcome. Acta Endocrinol 120:800–810
Eriksson RS, Thunberg L, Eriksson UJ (1989b) Effects of interrupted insulin treatment on fetal outcome of pregnant diabetic rats. Diabetes 38:764–772
Eshkind LG, Leube RE (1995) Mice lacking synaptophysin reproduce and form typical synaptic vesicles. Cell Tissue Res 282:423–433
Fagnou DD, Tuchek JM (1995) The biochemistry of learning and memory. Mol Cell Biochem 149:279–286
Farooq M, Ayaz A, Bahoo A, Ahmad I (2007) Maternal and neonatal outcomes in gestational diabetes mellitus. Int J Endocrinol Metab 2007:109–115
Fetita L-S, Sobngwi E, Serradas P, Calvo F, Gautier J-F (2006) Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 91:3718–3724
Frick KM, Fernandez SM (2003) Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging 24:615–626
Frick K, Fernandez S, Bulinski S (2002) Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115:547–558
Garner CC, Zhai RG, Gundelfinger ED, Ziv NE (2002) Molecular mechanisms of CNS synaptogenesis. Trends Neurosci 25:243–250
Ge X et al. (2015) Development of the human fetal hippocampal formation during early second trimester. NeuroImage 119:33–43
Georgieff MK (2006) The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minn Med 89:44–47
Gincel D, Shoshan-Barmatz V (2002) The synaptic vesicle protein synaptophysin: purification and characterization of its channel activity. Biophys J 83:3223–3229
Gispen WH, Biessels G-J (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23:542–549
Haghir H, Nomani H, Sankian M, Kheradmand H, Hami J (2013a) Sexual dimorphism in expression of insulin and insulin-like growth factor-I receptors in developing rat cerebellum. Cell Mol Neurobiol 33:369–377
Haghir H, Sankian M, Kheradmand H, Hami J (2013b) The effects of induced type-I diabetes on developmental regulation of insulin & insulin like growth factor-1 (IGF-1) receptors in the cerebellum of rat neonates. Metab Brain Dis 28:397–410
Hami J, Sadr-Nabavi A, Sankian M, Balali-Mood M, Haghir H (2013) The effects of maternal diabetes on expression of insulin-like growth factor-1 and insulin receptors in male developing rat hippocampus. Brain Struct Funct 218:73–84
Hami J, Karimi R, Haghir H, Gholamin M, Sadr-Nabavi A (2015a) Diabetes in pregnancy adversely affects the expression of glycogen synthase kinase-3beta in the hippocampus of rat neonates. J Mol Neurosci 57:273–281. doi:10.1007/s12031-015-0617-3
Hami J, Kerachian MA, Karimi R, Haghir H, Sadr-Nabavi A (2015b) Effects of streptozotocin-induced type 1 maternal diabetes on PI3K/AKT signaling pathway in the hippocampus of rat neonates. J Recept Signal Transduct Res 1–7 doi:10.3109/10799893.2015.1086884
Hami J, Shojae F, Vafaee-Nezhad S, Lotfi N, Kheradmand H, Haghir H (2015c) Some of the experimental and clinical aspects of the effects of the maternal diabetes on developing hippocampus. World J Diabetes 6:412
Hami J, Vafaei-Nezhad S, Haghir D, Haghir H (2015d) Insulin-Like Growth Factor-1 Receptor Is Differentially Distributed in Developing Cerebellar Cortex of Rats Born to Diabetic Mothers Journal of molecular neuroscience: MN
Hami J, Vafaei-Nezhad S, Ghaemi K, Sadeghi A, Ivar G, Shojae F, Hosseini M (2016) Stereological study of the effects of maternal diabetes on cerebellar cortex development in rat. Metab Brain Dis. doi:10.1007/s11011-016-9802-5
Hans J, Lammens M (2009) Development of the human cerebellum and its disorders. Clin Perinatol 36:513–530
Hans J, Lammens M, Wesseling P, Hori A (2014) Development and developmental disorders of the human cerebellum. In: Clinical neuroembryology. Springer, pp 371–420
Harada A, Sobue K, Hirokawa N (1990) Developmental changes of synapsin I subcellular localization in rat cerebellar neurons. Cell Struct Funct 15:329–342
Heinonen O, Soininen H, Sorvari H, Kosunen O, Palja L, Koivisto E, Riekkinen P (1995) Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience 64:375–384
Hibi M, Shimizu T (2012) Development of the cerebellum and cerebellar neural circuits. Dev Neurobiol 72:282–301
Ho N, Sommers MS, Lucki I (2013) Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 37:1346–1362
Humphrey T (1967) The development of the human hippocampal fissure. J Anat 101:655–676
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178
Isohanni M et al. (2001) Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res 52:1–19
Jackson-Guilford J, Leander JD, Nisenbaum LK (2000) The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett 293:91–94
Jahn R, Schiebler W, Ouimet C, Greengard P (1985) A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci 82:4137–4141
Janz R, Südhof TC, Hammer RE, Unni V, Siegelbaum SA, Bolshakov VY (1999) Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I Neuron 24:687–700
Jawerbaum A, White V (2010) Animal models in diabetes and pregnancy. Endocr Rev 31:680–701. doi:10.1210/er.2009-0038
Joca SR, Guimarães FS, Del-Bel E (2007) Inhibition of nitric oxide synthase increases synaptophysin mRNA expression in the hippocampal formation of rats. Neurosci Lett 421:72–76
Johnston P, Südhof T (1990) The multisubunit structure of synaptophysin. Relationship between disulfide bonding and homo-oligomerization. J Biol Chem 265:8869–8873
Kamal A, Biessels G-J, Urban I, Gispen W (1999) Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience 90:737–745
Khaksar Z, Jelodar G, Hematian H (2010) Effect of Maternal Diabetes on Cerebellum Histomorphometry in Neonatal Rats. Shahid Sadoughi Univ Med Sci 18:56–63
Lapolla A, Dalfra MG, Fedele D (2005) Insulin therapy in pregnancy complicated by diabetes: are insulin analogs a new tool? Diabetes Metab Res Rev 21:241–252. doi:10.1002/dmrr.551
Leclerc N, Beesley PW, Brown I, Colonnier M, Gurd JW, Paladino T, Hawkes R (1989) Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol 280:197–212
Li L, Tasic B, Micheva KD, Ivanov VM, Spletter ML, Smith SJ, Luo L (2010) Visualizing the distribution of synapses from individual neurons in the mouse brain. PLoS One 5:e11503–e11503
Lu CS, Van Vactor D (2013) Chapter 29 - Genetic Analysis of Synaptogenesis. In: Rakic JLRR (ed) Cellular Migration and Formation of Neuronal Connections. Academic Press, Oxford, pp 537–577. doi:10.1016/B978-0-12-397266-8.00104-6
Magaton A, Gil FZ, Casarini DE, de Fatima CM, Gomes GN (2007) Maternal diabetes mellitus-early consequences for the offspring. Pediatr Nephrol 22:37–43
Marazzi G, Buckley KM (1993) Accumulation of mRNAs encoding synaptic vesicle-specific proteins precedes neurite extension during early neuronal development. Dev Dyn 197:115–124
Masliah E, Terry RD, DeTeresa RM, Hansen LA (1989) Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett 103:234–239
McGahon B, Clements M, Lynch M (1997) The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience 81:9–16
McMahon HT, Bolshakov VY, Janz R, Hammer RE, Siegelbaum SA, Südhof TC (1996) Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc Natl Acad Sci 93:4760–4764
Okouchi M, Okayama N, Aw TY (2005) Differential susceptibility of naive and differentiated PC-12 cells to methylglyoxal-induced apoptosis: influence of cellular redox. Curr Neurovasc Res 2:13–22
Ornoy A, Ratzon N, Greenbaum C, Peretz E, Soriano D, Dulitzky M (1998) Neurobehaviour of school age children born to diabetic mothers. Arch Dis Childhood-Fetal Neonatal Ed 79:F94–F99
Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M (2001) School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab 14:681–690
Ozcelik T, Lafreniere R, Archer B 3rd, Johnston P, Willard H, Francke U, Südhof T (1990) Synaptophysin: structure of the human gene and assignment to the X chromosome in man and mouse. Am J Hum Genet 47:551
Petzoldt AG, Sigrist SJ (2014) Synaptogenesis. Curr Biol 24:R1076–R1080. doi:10.1016/j.cub.2014.10.024
Popoviç M, Biessels G-J, Isaacson RL, Gispen WH (2001) Learning and memory in streptozotocin-induced diabetic rats in a novel spatial/object discrimination task. Behav Brain Res 122:201–207
Pyeon H-J, Lee Y-I (2012) Differential expression levels of synaptophysin through developmental stages in hippocampal region of mouse brain. Anat Cell Biol 45:97–102
Rizzo T, Metzger BE, Burns WJ, Burns K (1991) Correlations between antepartum maternal metabolism and intelligence of offspring New England. J Med 325:911–916
Rizzo T, Silverman B, Metzger B, Cho N (1997) Behavioral adjustment in children of diabetic mothers. Acta Paediatr 86:969–974
Salvesen DR, Freeman J, Brudenell JM, Nicolaides KH (1993) Prediction of fetal acidemia in pregnancies complicated by maternal diabetes-mellitus by biophysical profile scoring and fetal heart-rate monitoring brit. J Obstet Gynaecol 100:227–233. doi:10.1111/j.1471-0528.1993.tb15235.x
Schwartz R, Teramo KA (2000) Effects of diabetic pregnancy on the fetus and newborn. In: Semin Perinatol, vol 2. Elsevier, pp 120–135
Schwartz R, Gruppuso PA, Petzold K, Brambilla D, Hiilesmaa V, Teramo KA (1994) Hyperinsulinemia and Macrosomia in the Fetus of the Diabetic Mother. Diabetes Care 17:640–648. doi:10.2337/diacare.17.7.640
Shin B-C, Fujikura K, Suzuki T, Tanaka S, Takata K (1997) Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology 138:3997–4004
Silverman BL et al. (1991) Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 40:121–125
Siman C, Eriksson U (1997) Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia 40:1416–1424
Simán CM, Eriksson UJ (1997) Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes 46:1054–1061
Singh BS, Westfall TC, Devaskar SU (1997) Maternal Diabetes-Induced Hyperglycemia and Acute Intracerebral Hyperinsulinism Suppress Fetal Brain Neuropeptide Y Concentrations 1. Endocrinology 138:963–969
Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20:327–348
Styrud J, Thunberg L, Nybacka O, Eriksson U (1995) Correlations between maternal metabolism and deranged development in the offspring of normal and diabetic rats. Pediatr Res 37:343–353
Suzuki T, Yao W (2014) Molecular and structural bases for postsynaptic signal processing: interaction between postsynaptic density and postsynaptic membrane rafts. J Neurorestoratology 2:1–14
Suzuki N, Svensson K, Eriksson U (1996) High glucose concentration inhibits migration of rat cranial neural crest cells in vitro. Diabetologia 39:401–411
Sze C-I, Troncoso JC, Kawas C, MOUTION P, Price DL, Martin LJ (1997) Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. Journal of Neuropathology & Exp Neurol 56:933–944
Takata K, Fujikura K, Shin B-C (1997) Ultrastructure of the rodent placental labyrinth: a site of barrier and transport. J Reprod Dev 43:13–24
Thach WT, Goodkin H, Keating J (1992) The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15:403–442
Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS, Henn FA (2001) Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry 50:809–812
Turner BM, Paradiso S, Marvel CL, Pierson R, Ponto LLB, Hichwa RD, Robinson RG (2007) The cerebellum and emotional experience. Neuropsychologia 45:1331–1341
Vafaei-Nezhad S, Hami J, Sadeghi A, Ghaemi K, Hosseini M, Abedini MR, Haghir H (2016) The Impacts of Diabetes in Pregnancy on Hippocampal Synaptogenesis in Rat Neonates. Neuroscience 318:122–133. doi:10.1016/j.neuroscience.2016.01.025
Valtorta F, Pennuto M, Bonanomi D, Benfenati F (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis?. BioEssays 26:445–453 doi:10.1002/bies.20012
Waites CL, Craig AM, Garner CC (2005) Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci 28:251–274
Wentzel P, Thunberg L, Eriksson UJ (1997) Teratogenic effect of diabetic serum is prevented by supplementation of superoxide dismutase and N-acetylcysteine in rat embryo culture. Diabetologia 40:7–14
Yamashita Y, Kawano Y, Kuriya N, Murakami Y, Matsuishi T, Yoshimatsu K, Kato H (1996) Intellectual development of offspring of diabetic mothers. Acta Paediatr 85:1192–1196
Zhan S-S, Beyreuther K, Schmitt H (1993) Quantitative assessment of the synaptophysin immuno-reactivity of the cortical neuropil in various neurodegenerative disorders with dementia. Dement Geriatr Cogn Disord 4:66–74
Zhou J, Wang L, Ling S, Zhang X (2007) Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp Neurol 206:201–208. doi:10.1016/j.expneurol.2007.04.013
Acknowledgments
This study was financially supported by Birjand University of Medical Sciences (BUMS) Grant (No. 1027). The authors gratefully thank to Prof. Ebrahim Esfandiari, Prof. Mohammad Mardani, Prof. Mohammad Afshar, Dr. Mohammad Mahdi Hasanzadeh Taheri, Prof. Shahnaz Razavi, Dr. Mohammad Fereidooni, and Mrs. Nassim Lotfi for their technical assistance and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no financial or nonfinancial conflicts of interest to declare
Rights and permissions
About this article
Cite this article
Hami, J., Vafaei-Nezhad, S., Ivar, G. et al. Altered expression and localization of synaptophysin in developing cerebellar cortex of neonatal rats due to maternal diabetes mellitus. Metab Brain Dis 31, 1369–1380 (2016). https://doi.org/10.1007/s11011-016-9864-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9864-4