Abstract
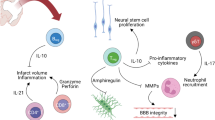
Peroxisome proliferator-activated receptor alpha (PPARα) is a nuclear receptor transcription factor that plays a role in immune regulation. Because of its expression in cerebral tissue and immune cells, PPARα has been examined as an important regulator in immune-based neurological diseases. Many studies have indicated that pre-treatment of animals with PPARα agonists induces protection against stroke. However, our previous reports indicate that protection is only in males, not females, and can be attributed to different PPARα expression between the sexes. In the current study, we examine how loss of PPARα affects male and female mice in experimental stroke. Male and female PPARα knockout mice were subject to middle cerebral artery occlusion (MCAO) or sham surgery, and the ischemic (local) or spleen specific (peripheral) immune response was examined 96 h after reperfusion. We found that loss of PPARα perpetuated sex differences in stroke, and this was driven by the peripheral, not local, immune response. Specifically we observed an increase in peripheral pro-inflammatory and adhesion molecule gene expression in PPARα KO males after MCAO compared to females. Our data supports previous evidence that PPARα plays an important role in sex differences in the immune response to disease, including stroke.







Similar content being viewed by others
Abbreviations
- PPARα:
-
Peroxisome proliferator-activated receptor alpha
- MCAO:
-
Middle cerebral artery occlusion
- Treg:
-
Regulatory T cell
References
Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H (2013) Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res 4:554–563. doi:10.1007/s12975-013-0268-z
Bishop-Bailey D (2000) Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol 129:823–834. doi:10.1038/sj.bjp.0703149
Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, −beta, and -gamma in the adult rat. Endocrinology 137:354–366. doi:10.1210/endo.137.1.8536636
Chinetti G et al (1998) Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem 273:25573–25580
Collino M et al (2006) Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med 41:579–589. doi:10.1016/j.freeradbiomed.2006.04.030
Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB (1998) Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem 70:1366–1375
Delerive P, Fruchart JC, Staels B (2001) Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169:453–459
Deplanque D et al (2003) Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci Off J Soc Neurosc 23:6264–6271
Di Carlo A et al (2003) Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 34:1114–1119. doi:10.1161/01.str.0000068410.07397.d7
Dotson AL, Wang J, Chen Y, Manning D, Nguyen H, Saugstad JA, Offner H (2015a) Sex differences and the role of PPAR alpha in experimental stroke. Metab Brain Dis. doi:10.1007/s11011-015-9766-x
Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H (2015b) Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol 278:289–298. doi:10.1016/j.jneuroim.2014.11.020
Dunn SE et al (2007) Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med 204:321–330. doi:10.1084/jem.20061839
Ginsberg HN et al (2010) Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362:1563–1574. doi:10.1056/NEJMoa1001282
Guo Q, Wang G, Namura S (2010) Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 30:70–78. doi:10.1038/jcbfm.2009.185
Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H (2007) T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 27:1798–1805. doi:10.1038/sj.jcbfm.9600482
Jalouli M et al (2003) Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology 144:101–109. doi:10.1210/en.2002-220630
Jones DC, Ding X, Daynes RA (2002) Nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. J Biol Chem 277:6838–6845. doi:10.1074/jbc.M106908200
Kainu T, Wikstrom AC, Gustafsson JA, Pelto-Huikko M (1994) Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport 5:2481–2485
Leuenberger N, Pradervand S, Wahli W (2009) Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J Clin Invest 119:3138–3148. doi:10.1172/jci39019
Losey P, Ladds E, Laprais M, Geuvel B, Burns L, Bordet R, Anthony DC (2015) The role of PPAR activation during the systemic response to brain injury. J Neuroinflammation 12:99. doi:10.1186/s12974-015-0295-7
Marx N, Sukhova GK, Collins T, Libby P, Plutzky J (1999) PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 99:3125–3131
Moreno S, Farioli-Vecchioli S, Ceru MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145
Mozaffarian D et al (2015) Heart disease and stroke statistics--2015 update: a report from the american heart association. Circulation 131:e29–e322. doi:10.1161/cir.0000000000000152
Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD (2006a) Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 26:654–665. doi:10.1038/sj.jcbfm.9600217
Offner H et al (2006b) Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol (Baltimore, Md : 1950) 176:6523–6531
Ouk T et al (2014) Effects of the PPAR-alpha agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 34:542–551. doi:10.1038/jcbfm.2013.233
Poynter ME, Daynes RA (1998) Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem 273:32833–32841
Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR (2012a) A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J NeuroImmune Pharmacol 7:1017–1024. doi:10.1007/s11481-012-9406-8
Seifert HA et al (2012b) The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis 27:131–141. doi:10.1007/s11011-012-9283-0
Trottier J, Caron P, Straka RJ, Barbier O (2011) Profile of serum bile acids in noncholestatic volunteers: gender-related differences in response to fenofibrate. Clin Pharmacol Ther 90:279–286. doi:10.1038/clpt.2011.124
Turner RC, Lucke-Wold B, Lucke-Wold N, Elliott AS, Logsdon AF, Rosen CL, Huber JD (2013) Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. Int J Mol Sci 14:1890–1917. doi:10.3390/ijms14011890
Wege N et al (2015) Men and women differ in their diurnal expression of monocyte peroxisome proliferator-activated receptor-alpha in the fed but not in the fasted state. FASEB J Off Publ Fed Am Soc Exp Biol 29:2905–2911. doi:10.1096/fj.14-267575
Yessoufou A, Hichami A, Besnard P, Moutairou K, Khan NA (2006) Peroxisome proliferator-activated receptor alpha deficiency increases the risk of maternal abortion and neonatal mortality in murine pregnancy with or without diabetes mellitus: modulation of T cell differentiation. Endocrinology 147:4410–4418. doi:10.1210/en.2006-0067
Zhang W et al (2008) Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39:2073–2078. doi:10.1161/strokeaha.107.508325
Acknowledgements
The authors wish to thank Gail Kent for assistance with manuscript submission. This work was supported by NIH/NINDS 5R01NS076013 (HO, JAS). This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Oregon Health & Science University’s Institutional Animal Care and Use Committee.
This article does not contain any studies with human participants performed by any of the authors.
Funding
This work was supported by NIH/NINDS 5R01NS076013 (HO, JAS). This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dotson, A.L., Wang, J., Liang, J. et al. Loss of PPARα perpetuates sex differences in stroke reflected by peripheral immune mechanisms. Metab Brain Dis 31, 683–692 (2016). https://doi.org/10.1007/s11011-016-9805-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9805-2