Abstract
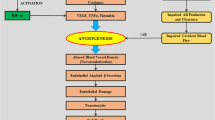
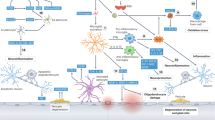
Vascular dementia (VaD) is the second most common cause of dementia and leads to a decline in cognitive thinking via conditions that lead to blockage or reduced blood flow to the brain. It is a poorly understood disease, and the changes that occur are often linked to other types of dementia such as Alzheimer’s disease. To date, there are no approved therapies or drugs to treat the symptoms of VaD, even though there is some evidence of drugs approved for Alzheimer’s that might have some benefit in patients diagnosed with VaD. The altered blood flow that precedes VaD may result in compensatory mechanisms, such as angiogenesis, to increase blood flow in the brain. Angiogenesis, the process of new blood vessel formations from pre-existing ones, involves several pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and is regulated by a variety of growth factors from neurons, astrocytes, and pericytes in the brain as well the extracellular matrix (ECM). The ECM highly regulates angiogenesis and other processes in the brain. One such ECM component is the heparan sulfate proteoglycan perlecan and its bioactive region, Domain V (DV). Here we discuss the potential role of DV as a novel therapy to treat VaD.

Similar content being viewed by others
References
Arikawa-Hirasawa E et al (1999) Perlecan is essential for cartilage and cephalic development. Nat Genet 23:354–358
Bix G et al (2004) Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J Cell Biol 166(1):97–109
Bix G et al (2013) Perlecan domain V is neuroprotective and affords functional improvement in a photothrombotic stroke model in young and aged mice. Transl Stroke Res 5(5):515–23
Caplan L, Gomes J (2010) Binswanger disease—an update. J Neurol Sci 299:9–10
Clarke D et al (2012) Perlecan domain v induces VEGF secretion in brain endothelial cells through integrin α5β1 and ERK-dependent signaling pathways. PLoS One 7(9):e45257
Costell M et al (1999) Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 147(5):1109–1122
Farach-Carson M, Carson D (2007) Perlecan: a multifunctional extracellular proteoglycan scaffold. Glycobiology 17(9):897–905
Ferrer I (2010) Cognitive impairment of vascular origin: Neuropathology of cognitive impairment of vascular origin. J Neurol Sci 299:139–149
Fujita Y et al (2010) Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke 41(12):2938–43
Fukuda S et al (2004) Focal cerebral ischemia induces active proteinases that degrade microvascular matrix. Stroke 35(4):998–1004
Ghiso J et al (2010) Cerebral amyloid angiopathy and Alzheimer’s disease. Hirosaki Igaku 61:S111–124
Goyal A et al (2011) Endorepellin, the angiostatin module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): A dual receptor antagonism. J Biol Chem 286(29):25947–62
Harris J et al (2000) Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. PNAS 97(14):7754–59
Hooijmans C et al (2012) The effects of long-term omega-3 fatty acid supplementation of cognition and Alzheimer’s pathology in animals models of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 28(1):191–209
Iadecola C et al (2009) Threats to the mind: aging, amyloid, and hypertension. Stroke 40(3):S40–4
Iemolo F, et al (2009) Pathophysiology of vascular dementia. Immunity and Aging 6(13)
Iozzo R, San Antonio J (2001) Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest 108(3):349–355
Jellinger K (2013) Pathology and pathogenesis of vascular cognitive impairment—a critical update. Front Aging Neurosci 5:17
Jiang X et al (2004) Essential contribution of tumor-derived perlecan to epidermal tumor growth and angiogenesis. J Histochem Cytochem 52(12):1575–90
Kahle P et al (2012) Perlecan domain V is upregulated in human brain arteriovenous malformation and could mediate the vascular endothelial growth factor effect in lesional tissue. Neuroreport 23(10):627–30
Kravitz E et al (2012) Cognitive decline and dementia in the oldest-old. Rambam Maimonides Med J 3(4):e0026
Lee B et al (2011) Perlecan domain v is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest 121(8):3005–23
Lin B (2013) Encephalopathy: a vicious cascade following forebrain ischemia and hypoxia. Cent Nerv Syst Agents Med Chem 13:57–70
Maki T et al (2011) Angiogenic roles of adrenomedullin through vascular endothelial growth factor induction. Neuroreport 22(9):442–7
Milner R et al (2008) Increased expression of fibronectin and the α5β1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci 38(1):43–52
Mongiat M et al (2003) Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem 278(6):4238–49
Parham C et al (2014) Perlecan domain v inhibits amyloid-beta induced brain endothelial cell toxicity and restores angiogenic function. J Alzheimers Dis 38(2):415–23
Roberts J et al (2012) Perlecan and the blood-brain barrier: beneficial proteolysis? Front Pharmacol 3:155
Rocca W, Kokmen E (1999) Frequency and distribution of vascular dementia. Alzheimer Dis Assoc Disord 13(3):S9–14
Rodgers K et al (2007) Reduced perlecan in mice results in chondrodysplasia resembling Schwartz-Jampel syndrome. Hum Mol Genet 16(5):515–28
Saini M, Bix G (2012) Oxygen-glucose deprivation (OGD) and interleukin-1 (IL-differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain Res 1438:65–74
Shee W, Ong W, Lim T (1998) Distribution of perlecan in mouse hippocampus following intracerebroventricular kainite injections. Brain Res 799(2):292–300
Shibata M et al (2004) White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 35(11):2598–603
Thal D et al (2012) Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47(11):816–24
Van Horssen J et al (2001) Heparan sulfate proteoglycan expression in cerebrovascular amyloid beta deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol 102(6):604–16
Viswanathan A, Chabriat H (2006) Cerebral microhemorrhage. Stroke 37(2):550–5
Whitelock J et al (1996) The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem 271(17):10079–86
Wiesmann M et al (2013) Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab 33(11):1696–706
Willis C et al (2013) Endorepellin laminin-like globular 1/2 domains bind Ig3-5 of vascular endothelial growth factor (VEGF) receptor 2 and block pro-angiogenic signaling by VEGFA in endothelial cells. FEBS J 280(10):2271–84
Wright S et al (2012) Perlecan domain V inhibits α2 integin-mediated amyloid-β neurotoxicity. Neurobiol Aging 33(7):1379–88
Yamada K et al (2010) Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer’s disease. Brain Res 1352:176–86
Zhao G et al (2003) Temporal profile of tissue plasminogen activator (tPA) and inhibitor expression after transient focal cerebral ischemia. Neuroreport 14(13):1689–92
Acknowledgments
The authors would like to thank Dr. Jill Roberts and Leon de Hoog for their helpful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marcelo, A., Bix, G. The potential role of perlecan domain V as novel therapy in vascular dementia. Metab Brain Dis 30, 1–5 (2015). https://doi.org/10.1007/s11011-014-9576-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-014-9576-6