Abstract
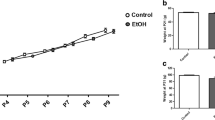
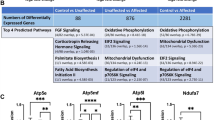
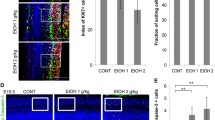
Fetal alcohol spectrum disorders (FASD) results from ethanol exposure to the developing fetus and is the leading cause of mental retardation. FASD is associated with a broad range of neurobehavioral deficits which may be mediated by ethanol-induced neurodegeneration in the developing brain. An immature brain is more susceptible to ethanol neurotoxicity. We hypothesize that the enhanced sensitivity of the immature brain to ethanol is due to a limited capacity to alleviate cellular stress. Using a third trimester equivalent mouse model of ethanol exposure, we demonstrated that subcutaneous injection of ethanol induced a wide-spread neuroapoptosis in postnatal day 4 (PD4) C57BL/6 mice, but had little effect on the brain of PD12 mice. We analyzed the expression profile of genes regulating apoptosis, and the pathways of ER stress response (also known as unfolded protein response, UPR) and autophagy during these ethanol-sensitive and resistant periods (PD4 versus PD12) using PCR microarray. The expression of pro-apoptotic genes, such as caspase-3, was much higher on PD4 than PD12; in contrast, the expression of genes that regulate UPR and autophagy, such as atf6, atg4, atg9, atg10, beclin1, bnip3, cebpb, ctsb, ctsd, ctss, grp78, ire1α, lamp, lc3 perk, pik3c3, and sqstm1 was significantly higher on PD12 than PD4. These results suggest that the vulnerability of the immature brain to ethanol could result from high expression of pro-apoptotic proteins and a deficiency in the stress responsive system, such as UPR and autophagy.



Similar content being viewed by others
References
Chen G, Luo J (2010) Anthocyanins: are they beneficial in treating ethanol neurotoxicity? Neurotox Res 17(1):91–101
Chen G, Ke Z, Xu M, Liao M, Wang X, Qi Y, Zhang T, Frank JA, Bower KA, Shi X, Luo J (2012) Autophagy is a protective response to ethanol neurotoxicity. Autophagy 8(11):1577–1589
Fernando P, Brunette S, Megeney LA (2005) Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J 19(12):1671–1673
Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G (2008) To die or not to die: that is the autophagic question. Curr Mol Med 8(2):78–91
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221(1):3–12
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Hellemans KG, Sliwowska JH, Verma P, Weinberg J (2010) Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev 34(6):791–807
Ikonomidou C (2009) Triggers of apoptosis in the immature brain. Brain Dev 31(7):488–492
Ke Z, Wang X, Liu Y, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Li M, Fang S, Shi X, Luo J (2011a) Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin Exp Res 35(9):1574–1583
Ke Z, Liu Y, Wang X, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Ou X, Shi X, Luo J (2011b) Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J Neurosci Res 89(10):1676–1684
Khan JY, Black SM (2003) Developmental changes in murine brain antioxidant enzymes. Pediatr Res 54(1):77–82
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke ZJ, Luo J (2009) Overexpression of glycogen synthase kinase 3beta sensitizes neuronal cells to ethanol toxicity. J Neurosci Res 87(12):2793–2802
Luo J (2012) Mechanisms of ethanol-induced death of cerebellar granule cells. Cerebellum 11(1):145–154
Madalosso SH, Pérez-Villegas EM, Armengol JA (2005) Naturally occurring neuronal death during the postnatal development of Purkinje cells and their precerebellar afferent projections. Brain Res Brain Res Rev 49(2):267–279
Maier SE, Miller JA, Blackwell JM, West JR (1999) Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res 23(4):726–734
Mocchetti I, Bachis A, Masliah E (2008) Chemokine receptors and neurotrophic factors: potential therapy against aids dementia? J Neurosci Res 86(2):243–255
Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D'Sa C, Roth KA (2002) Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis 9(2):205–219
Oomman S, Finckbone V, Dertien J, Attridge J, Henne W, Medina M, Mansouri B, Singh H, Strahlendorf H, Strahlendorf J (2004) Active caspase-3 expression during postnatal development of rat cerebellum is not systematically or consistently associated with apoptosis. J Comp Neurol 476(2):154–173
Rasheva VI, Domingos PM (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14:996–1007
Riley EP, Infante MA, Warren KR (2011) Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev 21(2):73–80
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1383–1388
Shen HM, Codogno P (2011) Autophagic cell death: Loch Ness monster or endangered species? Autophagy 7(5):457–465
Shimoji M, Pagan F, Healton EB, Mocchetti I (2009) CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotox Res 16(3):318–328
Siler-Marsiglio KI, Madorsky I, Pan Q, Paiva M, Neeley AW, Shaw G, Heaton MB (2006) Effects of acute ethanol exposure on regulatory mechanisms of Bcl-2-associated apoptosis promoter, bad, in neonatal rat cerebellum: differential effects during vulnerable and resistant developmental periods. Alcohol Clin Exp Res 30(6):1031–1038
Spreafico R, Frassoni C, Arcelli P, Selvaggio M, De Biasi S (1995) In situ labeling of apoptotic cell death in the cerebral cortex and thalamus of rats during development. J Comp Neurol 363(2):281–295
Tiveron MC, Boutin C, Daou P, Moepps B, Cremer H (2010) Expression and function of CXCR7 in the mouse forebrain. J Neuroimmunol 224(1–2):72–79
Vidal RL, Hetz C (2012) Crosstalk between the UPR and autophagy pathway contributes to handling cellular stress in neurodegenerative disease. Autophagy 8(6):970–972
Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR, Lee AS (2010) Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ 17(3):488–498
Wang H, Bower KA, Frank JA, Xu M, Luo J (2013) Hypoxic preconditioning alleviates ethanol neurotoxicity: the involvement of autophagy. Neurotox Res
Wu Y, Peng H, Cui M, Whitney NP, Huang Y, Zheng JC (2009) CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem 109(4):1157–1167
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Yakovlev A, Khafizova M, Abdullaev Z, Loukinov D, Kondratyev A (2010) Epigenetic regulation of caspase-3 gene expression in rat brain development. Gene 450(1–2):103–108
Zhu Y, Matsumoto T, Mikami S, Nagasawa T, Murakami F (2009) SDF1/CXCR4 signalling regulates two distinct processes of precerebellar neuronal migration and its depletion leads to abnormal pontine nuclei formation. Development 136(11):1919–1928
Acknowledgments
This work was supported by grants from the National Institutes of Health (AA015407 and AA019693).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alimov, A., Wang, H., Liu, M. et al. Expression of autophagy and UPR genes in the developing brain during ethanol-sensitive and resistant periods. Metab Brain Dis 28, 667–676 (2013). https://doi.org/10.1007/s11011-013-9430-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9430-2