Abstract
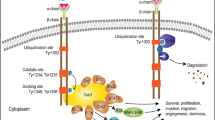
Glioblastoma is the most common aggressive, highly glycolytic, and lethal brain tumor. In fact, it is among the most commonly diagnosed lethal malignancies, with thousands of new cases reported in the United States each year. Glioblastoma’s lethality is derived from a number of factors including highly active pro-mitotic and pro-metastatic pathways. Two factors increasingly associated with the intracellular signaling and transcriptional machinery required for such changes are anaplastic lymphoma kinase (ALK) and the hepatocyte growth factor receptor (HGFR or, more commonly MET). Both receptors are members of the receptor tyrosine kinase (RTK) family, which has itself gained much attention for its role in modulating mitosis, migration, and survival in cancer cells. ALK was first described as a vital oncogene in lymphoma studies, but it has since been connected to many carcinomas, including non-small cell lung cancer and glioblastoma. As the receptor for HGF, MET has also been highly characterized and regulates numerous developmental and wound healing events which, when upregulated in cancer, can promote tumor progression. The wealth of information gathered over the last 30 years regarding these RTKs suggests three downstream cascades that depend upon activation of STAT3, Ras, and AKT. This review outlines the significance of ALK and MET as they relate to glioblastoma, explores the significance of STAT3, Ras, and AKT downstream of ALK/MET, and touches on the potential for new chemotherapeutics targeting ALK and MET to improve glioblastoma patient prognosis.




Similar content being viewed by others
Abbreviations
- ALK:
-
Anaplastic Lymphoma Kinase
- AKT:
-
Protein Kinase B
- ARF:
-
ADP Ribosylation Factor
- BAD:
-
Bcl-2 Associated Death promoter
- Bcl-2:
-
B-Cell Lymphoma 2
- BIM:
-
Bcl-2-like protein 11 gene
- c-myc:
-
Transcriptional Promoter Gene
- Cdc25A:
-
Cell Division Cycle 25 Homolog A
- CNS:
-
Central Nervous System
- EGFR:
-
Epidermal Growth Factor Receptor
- Elk-1:
-
E twenty-six-like transcription factor 1
- ERK:
-
Extracellular signal-Related Kinase
- FASL:
-
(CD95L) Fas Ligand gene
- FOXO:
-
Forkhead Box protein O1 (aka forkhead in rhabdomyosarcoma)
- GFAP:
-
Glial Fibrillary Acidic Protein
- Gsk3β:
-
Glycogen synthase kinase-3 (β isoform)
- HGF:
-
Hepatocyte Growth Factor
- HGFR:
-
(aka MET) Hepatocyte Growth Factor Receptor
- HIF-1α:
-
Hypoxia Inducible Factor
- IGF-1:
-
Insulin-like Growth Factor
- INK4a:
-
(aka CDKN2A) Cyclin-dependent kinase inhibitor 2a
- MDM2:
-
Murine Double Minute oncogene product
- MEK:
-
Mitogen-activated protein kinase kinase
- mTOR:
-
Mammalian Target of Rapamycin
- NBM:
-
Neuroblastoma
- NFκB:
-
Nuclear Factor kappa B
- PDGF:
-
Platelet Derived Growth Factor
- PI3K:
-
Phosphoinositide 3-kinase
- PIP2:
-
Phosphatidylinositol (4,5)-bisphosphate
- PIP3:
-
Phosphatidylinositol (3,4,5)-triphosphate
- PLT:
-
Pleiotrophin
- PNS:
-
Peripheral Nervous System
- PTEN:
-
Phosphatase and Tensin Homologue
- RAF:
-
Rapidly Accelerated Fibrosarcoma (viral gene)
- Ras:
-
GTPase dependent intracellular signal protein family
- RB1:
-
Retinoblastoma Protein 1
- RTK:
-
Receptor Tyrosine Kinase
- TMZ:
-
Temozolomide
- TP53:
-
Tumor Protein 53
- TSC2:
-
Tuberous Sclerosis Protein 2
- STAT3/5:
-
Signal Transducer and Activator of Transcription
- VEGF(R):
-
Vascular Endothelial Growth Factor (Receptor)
References
Aaronson DS, Horvath CM (2002) A road map for those who don’t know JAK-STAT. Science 296:1653–1655
Anandharaj A, Cinghu S, Park WY (2011) Rapamycin-mediated mTOR inhibition attenuates survivin and sensitizes glioblastoma cells to radiation therapy. Acta Biochim Biophys Sin 43:292–300
Arrieta O, Garcia E, Guevara P, Garcia-Navarrete R, Ondarza R, Rembao D, Sotelo J (2002) Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer 94:3210–3218
Bajbouj K, Mawrin C, Hartig R, Schulze-Luehrmann J, Wilisch-Neumann A, Roessner A, Schneider-Stock R (2012) P53-dependent antiproliferative and pro-apoptotic effects of trichostatin A (TSA) in glioblastoma cells. J Neuro-Oncol 107:503–516
Barre B, Vigneron A, Coqueret O (2005) The STAT3 transcription factor is a target for the Myc and riboblastoma proteins on the Cdc25A promoter. J Biol Chem 280:15673–15681
Batchelor TT, Duda DG, di Tomaso E et al (2010) Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol Off J Am Soc Clin Oncol 28:2817–2823
Benito R, Gil-Benso R, Quilis V, Perez M, Gregori-Romero M, Roldan P, Gonzalez-Darder J, Cerda-Nicolas M, Lopez-Gines C (2010) Primary glioblastomas with and without EGFR amplification: relationship to genetic alterations and clinicopathological features. Neuropathol Off J Japan Soc Neuropathol 30:392–400
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915–925
Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C (1995) Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376:768–771
Boufaied N, Wioland MA, Falardeau P, Gourdeau H (2010) TLN-4601, a novel anticancer agent, inhibits Ras signaling post Ras prenylation and before MEK activation. Anti Cancer Drugs 21:543–552
Campbell PM, Boufaied N, Fiordalisi JJ, Cox AD, Falardeau P, Der CJ, Gourdeau H (2010) TLN-4601 suppresses growth and induces apoptosis of pancreatic carcinoma cells through inhibition of Ras-ERK MAPK signaling. J Mol Signal 5:18
Cecchi F, Rabe DC, Bottaro DP (2010) Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer 46:1260–1270
Chen WS, Xu PZ, Gottlob K et al (2001) Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Gene Dev 15:2203–2208
Chi AS, Batchelor TT, Kwak EL, Clark JW, Wang DL, Wilner KD, Louis DN, Iafrate AJ (2012) Rapid radiographic and clinical improvement after treatment of a MET-amplified recurrent glioblastoma with a mesenchymal-epithelial transition inhibitor. J Clin Oncol Off J Am Soc Clin Oncol 30:e30–e33
Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, Karras JG, Levy DE, Inghirami G (2005) Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med 11:623–629
Chin LP, Soo RA, Soong R, Ou SH (2012) Targeting ROS1 with Anaplastic Lymphoma Kinase Inhibitors: A Promising Therapeutic Strategy for a Newly Defined Molecular Subset of Non-Small-Cell Lung Cancer. J Thorac Oncol 7:1625–1630
Christensen JG, Zou HY, Arango ME et al (2007) Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 6:3314–3322
Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF (1984) Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311:29–33
Cooper LA, Gutman DA, Chisolm C et al. (2012) The Tumor Microenvironment Strongly Impacts Master Transcriptional Regulators and Gene Expression Class of Glioblastoma. Am J Pathol
Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, Wilner KD (2011) CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol Off J Am Soc Clin Oncol 29:e443–e445
Crosswell HE, Dasgupta A, Alvarado CS, Watt T, Christensen JG, De P, Durden DL, Findley HW (2009) PHA665752, a small-molecule inhibitor of c-Met, inhibits hepatocyte growth factor-stimulated migration and proliferation of c-Met-positive neuroblastoma cells. BMC cancer 9:411
De Luca A, Maiello MR, D'Alessio A, Pergameno M and Normanno N (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets
de Pontual L, Kettaneh D, Gordon CT et al (2011) Germline gain-of-function mutations of ALK disrupt central nervous system development. Hum Mutat 32:272–276
Del Vecchio CA, Li G, Wong AJ (2012) Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Expet Rev Vaccine 11:133–144
Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM (1991) Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene 6:1997–2003
Dirks WG, Fahnrich S, Lis Y, Becker E, MacLeod RA, Drexler HG (2002) Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int J Cancer 100:49–56
Easton RM, Cho H, Roovers K et al (2005) Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 25:1869–1878
Faivre S, Kroemer G, Raymond E (2006) Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 5:671–688
Fan QW, Cheng C, Hackett C et al (2010) Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal 3:ra81
Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM (2011) Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neuro-Oncol 105:281–289
Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, Yamamoto T (1996) Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci U S A 93:4181–4186
Gariboldi MB, Ravizza R, Monti E (2010) The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem Pharmacol 80:455–462
Garofalo RS, Orena SJ, Rafidi K et al (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Investig 112:197–208
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G (2012) Targeting MET in cancer: rationale and progress. Nat Rev Canc 12:89–103
Goudar RK, Shi Q, Hjelmeland MD et al (2005) Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther 4:101–112
Grzelinski M, Steinberg F, Martens T, Czubayko F, Lamszus K, Aigner A (2009) Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia 11:145–156
Guessous F, Zhang Y, diPierro C, Marcinkiewicz L, Sarkaria J, Schiff D, Buchanan S, Abounader R (2010) An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth. Anti Cancer Agents Med Chem 10:28–35
Hagerstrand D, Lindh MB, Pena C, Garcia-Echeverria C, Nister M, Hofmann F, Ostman A (2010) PI3K/PTEN/Akt pathway status affects the sensitivity of high-grade glioma cell cultures to the insulin-like growth factor-1 receptor inhibitor NVP-AEW541. Neuro-Oncol 12:967–975
Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN (2000) Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 25:55–57
Houillier C, Lejeune J, Benouaich-Amiel A et al (2006) Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer 106:2218–2223
Hsieh A, Ellsworth R, Hsieh D (2011) Hedgehog/GLI1 regulates IGF dependent malignant behaviors in glioma stem cells. J Cell Physiol 226:1118–1127
Jin H, Yang R, Li W, Ogasawara AK, Schwall R, Eberhard DA, Zheng Z, Kahn D, Paoni NF (2003) Early treatment with hepatocyte growth factor improves cardiac function in experimental heart failure induced by myocardial infarction. J Pharmacol Exp Ther 304:654–660
Joo KM, Jin J, Kim E et al (2012) MET signaling regulates glioblastoma stem cells. Cancer Res 72:3828–3838
Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, Burgess TL (2007) AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res Off J Am Assoc Cancer Res 13:6735–6742
Kreisl TN, Lassman AB, Mischel PS, Rosen N, Scher HI, Teruya-Feldstein J, Shaffer D, Lis E, Abrey LE (2009) A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol 92:99–105
Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, Pomper MG, Kim J, Laterra J (2009) EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther 8:1751–1760
Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 24:7443–7454
Li J, Yen C, Liaw D et al (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947
Liu W, Fu Y, Xu S, Ding F, Zhao G, Zhang K, Du C, Pang B, Pang Q (2011) c-Met expression is associated with time to recurrence in patients with glioblastoma multiforme. J Clin Neurosci 18:119–121
Mason WP, Belanger K, Nicholas G, Vallieres I, Mathieu D, Kavan P, Desjardins A, Omuro A and Reymond D (2011) A phase II study of the Ras-MAPK signaling pathway inhibitor TLN-4601 in patients with glioblastoma at first progression. J Neuro Oncol
Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T (1999) STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 18:4261–4269
McDermott U, Iafrate AJ, Gray NS et al (2008) Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 68:3389–3395
McDowell KA, Riggins GJ, Gallia GL (2011) Targeting the AKT pathway in glioblastoma. Curr Pharm Des 17:2411–2420
Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276:60–66
Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT (1994) Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 263:1281–1284
Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR (2000) Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol 20:8969–8982
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453
Ohgaki H, Dessen P, Jourde B et al (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899
Pechkovsky DV, Prele CM, Wong J et al. (2012) STAT3-Mediated Signaling Dysregulates Lung Fibroblast-Myofibroblast Activation and Differentiation in UIP/IPF. Am J Pathol
Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M (2011) Conditional probability of survival in patients with newly diagnosed glioblastoma. J Clin Oncol Off J Am Soc Clin Oncol 29:4175–4180
Porter AC, Vaillancourt RR (1998) Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene 17:1343–1352
Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, Delsol G, Mason DY (1997) Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 89:1394–1404
Pulford K, Lamant L, Espinos E, Jiang Q, Xue L, Turturro F, Delsol G, Morris SW (2004a) The emerging normal and disease-related roles of anaplastic lymphoma kinase. Cell Mol Life Sci 61:2939–2953
Pulford K, Morris SW, Turturro F (2004b) Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol 199:330–358
Rao SK, Edwards J, Joshi AD, Siu IM, Riggins GJ (2010) A survey of glioblastoma genomic amplifications and deletions. J Neuro-Oncol 96:169–179
Samuel MS, Lourenco FC, Olson MF (2011) K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PLoS One 6:e17143
Schaper F, Siewert E, Gomez-Lechon MJ, Gatsios P, Sachs M, Birchmeier W, Heinrich PC, Castell J (1997) Hepatocyte growth factor/scatter factor (HGF/SF) signals via the STAT3/APRF transcription factor in human hepatoma cells and hepatocytes. FEBS Lett 405:99–103
Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C (1995) Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373:699–702
Shinojima N, Tada K, Shiraishi S et al (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63:6962–6970
Shirogane T, Fukada T, Muller JM, Shima DT, Hibi M, Hirano T (1999) Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity 11:709–719
Sinha S, Koul N, Dixit D, Sharma V, Sen E (2011) IGF-1 induced HIF-1alpha-TLR9 cross talk regulates inflammatory responses in glioma. Cell Signal 23:1869–1875
Sonnenberg E, Meyer D, Weidner KM, Birchmeier C (1993) Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 123:223–235
Staal SP, Hartley JW (1988) Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med 167:1259–1264
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stylianou DC, Auf der Maur A, Kodack DP, Henke RT, Hohn S, Toretsky JA, Riegel AT, Wellstein A (2009) Effect of single-chain antibody targeting of the ligand-binding domain in the anaplastic lymphoma kinase receptor. Oncogene 28:3296–3306
Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V, Mukhtar H (2008) Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol 231:52–60
Szerlip NJ, Pedraza A, Chakravarty D et al (2012) Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A 109:3041–3046
Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N (1995) Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373:702–705
Verhaak RG, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Vernersson E, Khoo NK, Henriksson ML, Roos G, Palmer RH, Hallberg B (2006) Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns 6:448–461
Vignot S, Faivre S, Aguirre D, Raymond E (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol Off J Eur Soc Med Oncol 16:525–537
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Canc 2:489–501
Ward MR, Agrotis A, Kanellakis P, Dilley R, Jennings G, Bobik A (1997) Inhibition of protein tyrosine kinases attenuates increases in expression of transforming growth factor-beta isoforms and their receptors following arterial injury. Arterioscler Thromb Vasc Biol 17:2461–2470
Weiss A, Brill B, Borghouts C, Delis N, Mack L and Groner B (2012) Survivin inhibition by an interacting recombinant peptide, derived from the human ferritin heavy chain, impedes tumor cell growth. J Cancer Res Clin Oncol
Welsh JW, Mahadevan D, Ellsworth R, Cooke L, Bearss D, Stea B (2009) The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol 4:69
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Wolf A, Agnihotri S, Guha A (2010) Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget 1:552–562
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B (1992) Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A 89:2965–2969
Xie Q, Bradley R, Kang L et al (2012) Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci U S A 109:570–575
Yamazaki S, Skaptason J, Romero D, Lee JH, Zou HY, Christensen JG, Koup JR, Smith BJ and Koudriakova T (2008) Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug metabolism and disposition: the biological fate of chemicals, 36, 1267–1274
Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, Levy DE, Inghirami G (2002) Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 21:1038–1047
Zeng Q, Chen S, You Z, Yang F, Carey TE, Saims D, Wang CY (2002) Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFkappa B. J Biol Chem 277:25203–25208
Zhang YW, Wang LM, Jove R, Vande Woude GF (2002) Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21:217–226
Acknowledgments
Completion of this project was made possible by funding from the National Institutes of Health (NIH) and National Institute of Neurological Disorders and Stroke (NINDS): (NS31622, NS-38146, NS-57811, and NS-41088), the State of South Carolina Spinal Cord Injury Research Project (SCSCIRF), VA (1I01BX001262-01A2), Pfizer, Inc., and the Jerry Zucker Fund for Brain Tumor Research of the MUSC Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallace, G.C., Dixon-Mah, Y.N., Vandergrift, W.A. et al. Targeting oncogenic ALK and MET: a promising therapeutic strategy for glioblastoma. Metab Brain Dis 28, 355–366 (2013). https://doi.org/10.1007/s11011-013-9401-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9401-7