Abstract
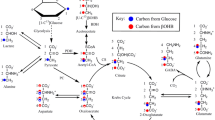
Hepatic encephalopathy is a severe neuropathological condition arising secondary to liver failure. The pathogenesis is not well understood; however, hyperammonemia is considered to be one causative factor. Hyperammonemia has been suggested to inhibit tricarboxylic acid (TCA) cycle activity, thus affecting energy metabolism. Furthermore, it has been suggested that catabolism of the branched-chain amino acid isoleucine may help curb the effect of hyperammonemia by by-passing the TCA cycle block as well as providing the carbon skeleton for glutamate and glutamine synthesis thus fixating ammonia. Here we present novel results describing [U-13C]isoleucine metabolism in muscle and brain analyzed by mass spectrometry in bile duct ligated rats, a model of chronic hepatic encephalopathy, and discuss them in relation to previously published results from neural cell cultures. The metabolism of [U-13C]isoleucine in muscle tissue was about five times higher than that in the brain which, in turn, was lower than in corresponding cell cultures. However, synthesis of glutamate and glutamine was supported by catabolism of isoleucine. In rat brain, differential labeling patterns in glutamate and glutamine suggest that isoleucine may primarily be metabolized in the astrocytic compartment which is in accordance with previous findings in neural cell cultures. Lastly, in rat brain the labeling patterns of glutamate, aspartate and GABA do not suggest any significant inhibition by ammonia of TCA cycle activity which corresponds well to findings in neural cell cultures. Branched-chain amino acids including isoleucine are used for treating hepatic encephalopathy and the present findings shed light on the possible mechanism involved. The low turn-over of isoleucine in rat brain suggests that this amino acid does not serve the role of providing metabolites pertinent to TCA cycle function and hence energy formation as well as the necessary carbon skeleton for subsequent ammonia fixation in hyperammonemia. The higher metabolism of isoleucine in muscle could, however, contribute to ammonia fixation and thus likely be of value in the treatment of hepatic encephalopathy.



Similar content being viewed by others
References
Albrecht J, Dolinska M (2001) Glutamine as a pathogenic factor in hepatic encephalopathy. J Neurosci Res 65:1–5
Biemann K (1962) Mass spectrometry. In: Organic chemistry applications. McGraw, New York, pp 223–227
Hawkins RA, Peterson DR, Viña JR (2002) The complementary membranes forming the blood-brain barrier. IUBMB Life 54:101–107
Hawkins RA, O’Kane RL, Simpson IA, Viña JR (2006) Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 136:218S–226S
Hindfelt B, Plum F, Duffy TE (1977) Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest 59:386–396
Iversen P, Sørensen M, Bak LK, Waagepetersen HS, Vafaee MS, Borghammer P, Mouridsen K, Jensen SB, Vilstrup H, Schousboe A, Ott P, Gjedde A, Keiding S (2009) Low cerebral oxygen consumption and blood flow in patients with cirrhosis and an acute episode of hepatic encephalopathy. Gastroenterology (in press)
Johansen ML, Bak LK, Schousboe A, Iversen P, Sørensen M, Keiding S, Vilstrup H, Gjedde A, Ott P, Waagepetersen HS (2007) The metabolic role of isoleucine in detoxification of ammonia in cultured mouse neurons and astrocytes. Neurochem Int 50:1042–1051
Lai JC, Cooper AJ (1986) Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J Neurochem 47:1376–1386
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U (2006) Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol 71:399–306
McKhann GM, Tower DB (1961) Ammonia toxicity and cerebral oxidative metabolism. Am J Physiol 200:420–424
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Ott P, Clemmesen O, Larsen FS (2005) Cerebral metabolic disturbances in the brain during acute liver failure: from hyperammonemia to energy failure and proteolysis. Neurochem Int 47:13–18
Rothe F, Brosz M, Storm-Mathisen J (1994) Quantitative ultrastructural localization of glutamate dehydrogenase in the rat cerebellar cortex. Neuroscience 62:1133–1146
Schousboe A, Waagepetersen HS (2007) Neuron-Glia interaction in homeostasis of the neurotransmitters glutamate and GABA. In: Malva JO, Rego AC, Cunha RA, Oliveira CR (eds) Interaction between neurons and Glia in aging and disease. Springer, New York, pp 111–120
Shawcross DL, Olde Damink SW, Butterworth RF, Jalan R (2005) Ammonia and hepatic encephalopathy: the more things change, the more the remain the same. Metab Brain Dis 20:169–179
van Anken HC, Schiphorst ME (1974) A kinetic determination of ammonia in plasma. Clin Chim Acta 56:151–157
Zaganas I, Waagepetersen HS, Georgopoulos P, Sonnewald U, Plaitakis A, Schousboe A (2001) Differential expression of glutamate dehydrogenase in cultured neurons and astrocytes from mouse cerebellum and cerebral cortex. J Neurosci Res 66:909–913
Zwingmann C, Chatauret N, Leibfritz D, Butterworth RF (2003) Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [H–C] nuclear magnetic resonance study. Hepatology 37:420–428
Acknowledgements
The expert secretarial assistance of Ms Hanne Danø and the skilful technical assistance of Ms Ann Lene Vigh are highly appreciated. The experimental work has been supported by grants from the Danish Medical Research Council (22-04-0314 and 271-07-0267) and the Lundbeck, Hørslev and Carlsberg Foundations. Professor Stephen Hamilton Dutoit, Institute of Pathology, Aarhus University Hospital is cordially thanked for help evaluating the liver biopsies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bak, L.K., Iversen, P., Sørensen, M. et al. Metabolic fate of isoleucine in a rat model of hepatic encephalopathy and in cultured neural cells exposed to ammonia. Metab Brain Dis 24, 135–145 (2009). https://doi.org/10.1007/s11011-008-9123-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-008-9123-4