Abstract
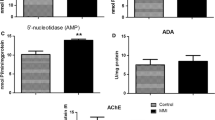
Thyroid hormones (THs) are recognized as key metabolic hormones, and the metabolic rate increases in hyperthyroidism, while it decreases in hypothyroidism. The aim of this work was to investigate how changes in metabolism induced by THs could affect the activities of acetylcholinesterase (AChE), (Na+, K+)- and Mg2+-ATPase in the hypothalamus and the cerebellum of adult rats. Hyperthyroidism was induced by subcutaneous administration of thyroxine (25μg/100 g body weight) once daily for 14 days, while hypothyroidism was induced by oral administration of propylthiouracil (0.05%) for 21 days. All enzyme activities were evaluated spectrophotometrically in the homogenated brain regions of 10 three-animal pools. Neither hyper-, nor hypothyroidism had any effect on the examined hypothalamic enzyme activities. In the cerebellum, hyperthyroidism provoked a significant decrease in both the AChE (−23%, p < 0.001) and the Na+, K+-ATPase activities (−26%, p < 0.001). Moreover, hypothyroidism had a similar effect on the examined enzyme activities: AChE (−17%, p < 0.001) and Na+, K+-ATPase (−27%, p < 0.001). Mg2+-ATPase activity was found unaltered in both the hyper- and the hypothyroid brain regions. In conclusion: neither hyper-, nor hypothyroidism had any effect on the examined hypothalamic enzyme activities. In the cerebellum, hyperthyroidism provoked a significant decrease in both the AChE and the Na+, K+-ATPase activities. The decreased (by the THs) Na+, K+-ATPase activities may increase the synaptic acetylcholine release, and thus, could result in a decrease in the cerebellar AChE activity. Moreover, the above TH-induced changes may affect the monoamine neurotransmitter systems.
Similar content being viewed by others
References
Adams RD, Rosman NP (1971) Hypothyroidism: neuromuscular system. In: Werner SC, Ingbar SH (eds). The thyroid. Academic Press, New York, pp. 901–910
Ahmed MT, Sinha AK, Pickard MR, Kim KD, Ekins RP (1993) Hypothyroidism in the adult rat causes brain region-specific biochemical dysfunction. J Endocrinol 138:299–305
Aminoff MJ, Greenberg DA, Simon RP (2005) Clinical neurology. Lange Medical Books/McGraw-Hill, New York, pp. 117–118
Atterwill CK, Reid J, Athayde CM (1985) Effect of thyroid status on the development of the different molecular forms of Na+,K+-ATPase in rat brain. Mol Cell Endocrinol 40:149–158
Bernal J, Nunez J (1995) Thyroid hormones and brain development. Eur J Endocrinol 133:390–398
Bogdanski DF, Tissari A, Brodie BB (1968) Role of sodium, potassium, ouabain and reserpine in uptake, storage and metabolism of biogenic amines in synaptosomes. Life Sci 7:419–428
Bowler K, Tirri R (1974) The temperature characteristics of synaptic membrane ATPases from immature and adult rat brain. J Neurochem 23:611–613
Bradley DJ, Young 3rd WS, Weinberger C (1989) Differential expression of alpha and beta thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad Sci USA 86:7250–7254
Carageorgiou H, Pantos C, Zarros A, Mourouzis I, Varonos D, Cokkinos D, Tsakiris S (2005) Changes in antioxidant status, protein concentration, acetylcholinesterase, (Na+, K+)- and Mg2+-ATPase activities in the brain of hyper- and hypothyroid adult rats. Metabol Brain Dis 20:129–139
Clos J, Ghandour S, Eberhart R, Vincendon G, Gombos G (1989) The cholinergic system in developing cerebellum: Comparative study of normal, hypothyroid and underfed rats. Dev Neurosci 11:188–204
Committee on Care and Use of Laboratory Animals (1985) Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources, National Research Council, Washington, DC, p. 83
Cook CB, Kakucska I, Lechan RM, Koenig RJ (1992) Expression of thyroid hormone receptor beta 2 in rat hypothalamus. Endocrinology 130:1077–1079
DeGroot LJ, Larsen PR, Refetoff S, Stanbury JB (1984) The thyroid and its diseases. John Wiley, Brisbane
Ellman GL, Courtney KD, Andres Jr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ernest S (1989) Model of gentamicin-induced nephrotoxicity and its amelioration by calcium and thyroxine. Med Hypotheses 30:195–202
Gullo D, Sinha AK, Woods R, Pervin K, Ekins RP (1987) Triiodothyronine binding in adult rat brain: compartmentation of receptor populations in purified neuronal and glial nuclei. Endocrinology 120:325–331
Hall RCW, Stickney S, Beresford TP (1986) Endocrine disease and behaviour. Integr Psychiat 4:122–135
Hernandez J (1987) Brain Na+, K+-ATPase activity possibly regulated by a specific serotonin receptor. Brain Res 408:399–402
Iversen S, Iversen L, Saper CB (2000) The autonomic nervous system and the hypothalamus. In: Kandel ER, Schwartz JH, Jessel TM (eds). Principles of neural science. McGraw-Hill, New York, pp. 960–981
Klegeris A, Korkina LG, Greenfield SA (1995) A possible interaction between acetylcholinesterase and dopamine molecules during autoxidation of the amine. Free Radic Biol Med 18:223–230
Kouniniotou-Krontiri P, Tsakiris S (1989) Time dependence of Li+ action on acetylcholinesterase activity in correlation with spontaneous quantal release of acetylcholine in rat diaphragm. Jnp J Physiol 39:429–440
Lees GJ, Lehmann A, Sandberg M, Hamberger A (1990) The neurotoxicity of ouabain, a sodium-potassium ATPase inhibitor, in the rat hippocampus. Neurosci Lett 120:159–162
Li Z, Yang R, Chen Z (1996) Effects of iodine and thyroid hormone deficiency during brain development on activities of cholinergic neuron-related enzymes in central nervous system of rats. Zhonghua Yu Fang Yi Xue Za Zhi 30:337–339
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mata M, Fink DJ, Gainer H, Smith CB, Davidsen L, Savakis H, Schwartz WJ, Sokoloff L (1980) Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem 34:213–215
Mellstrom B, Naranjo JR, Santos A, Gonzales AM, Bernal J (1991) Independent expression of the alpha and beta c-erbA genes in developing rat brain. Mol Endocrinol 5:1339–1350
Meyer ME, Cooper RJ (1981) Correlations between Na+, K+-ATPase activity and acetylcholine release in rat cortical synaptosomes. J Neurochem 36:467–475
Oppenheimer JH, Schwartz HL (1997) Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18:462–475
Pantos CI, Cokkinos DD, Tzeis SM, Malliopoulou V, Mourouzis IS, Carageorgiou HC, Limas C, Varonos D, Cokkinos DV (1999) Hyperthyroidism is associated with preserved preconditioning capacity but intensified and accelerated ischemic contracture in rat heart. Basic Res Cardiol 94:254–260
Pantos C, Malliopoulou V, Mourouzis I, Sfakianoudis K, Tzeis S, Doumba P, Xinaris C, Cokkinos AD, Carageorgiou H, Varonos DD, Cokkinos DV (2003) Propylthiouracil-induced hypothyroidism is associated with increased tolerance of the isolated rat heart to ischaemia-reperfusion. J Endocrinol 178:427–435
Patel AJ, Smith RM, Kingsbury AE, Hunt A, Balazs R (1980) Effects of thyroid state on brain development: muscarinic acetylcholine and GABA receptors. Brain Res 198:389–402
Rastogi RB, Hrdina PD, Dubas T, Singhal RL (1977) Alterations of brain acetylcholine metabolism during neonatal hyperthyroidism. Brain Res 123:188–192
Sanui H, Rubin H (1982) The role of magnesium in cell proliferation and transformation. In: Boynton AL, McKochan WL, Whitfield JP (eds). Ions cell proliferation and cancer. Academic Press, New York, pp. 517–537
Sastry BS, Phillis JW (1977) Antagonism of biogenic amine-induced depression of cerebral cortical neurones by Na+, K+-ATPase inhibitors. Can J Physiol Pharmacol 55:170–180
Swann AC (1984) (Na+, K+)-adenosine triphosphatase regulation by the sympathetic nervous system: Effects of noradrenergic stimulation and lesion in vivo. J Pharmacol Exp Ther 228:304–311
Tsakiris S (2001) Effects of L-phenylalanine on acetylcholinesterase and Na+, K+-ATPase activities in adult and aged rat brain. Mech Ageing Dev 122:491–501
Tsakiris S, Angelogianni P, Schulpis KH, Behrakis P (2000) Protective effect of L-cysteine and glutathione on rat brain Na+, K+-ATPase inhibition induced by free radicals. Z Naturforsch [C] 55:271–277
Virgili M, Saverino O, Vaccari M, Barnabei O, Contestabile A (1991) Temporal, regional and cellular selectivity of neonatal alteration of the thyroid state on neurochemical maturation in the rat. Exp Brain Res 83:555–561
Acknowledgements
This work was funded by the University of Athens and the University of Ioannina. Many thanks are expressed to the medical students Elena Gkrouzman, Zois Mellios and Christina Katsioni for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carageorgiou, H., Pantos, C., Zarros, A. et al. Effects of hyper- and hypothyroidism on acetylcholinesterase, (Na+, K+)- and Mg 2+-ATPase activities of adult rat hypothalamus and cerebellum. Metab Brain Dis 22, 31–38 (2007). https://doi.org/10.1007/s11011-006-9034-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-006-9034-1