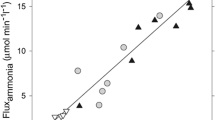
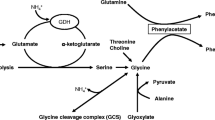
Abstract
Disturbed body nitrogen homeostasis due to impaired hepatic urea synthesis leads to an alteration in interorgan ammonia trafficking, resulting in hyperammonemia. Glutamine (Gln) synthase is the alternative pathway for ammonia detoxification. Gln taken up by several organs is split by the intramitochondrial phosphate-activated enzyme glutaminase (PAG) into glutamate (Glu) and ammonia. In cirrhotic patients with portosystemic intrahepatic shunt, the main source of systemic hyperammonemia is the small intestine, and ammonia derives mainly from Gln deamidation. Recently, PAG has been found increased in cirrhotics showing minimal hepatic encephalopathy and, therefore, could be implicated in the production of systemic hyperammonemia in these patients. Intestinal PAG activity correlates with psychometric test and magnetic resonance spectroscopy findings. Moreover, nitric oxide and tumor necrosis factor seem to be the major factors regulating intestinal ammonia production in cirrhotics. In the brain, PAG localized into the astrocytes is responsible for ammonia and free-radical production. The blockade of PAG, using 6-oxo-5-norleucine, avoids the toxic effects of Gln accumulation in the brain. These data support an important role for intestinal and brain glutaminase in the pathogenesis of hepatic encephalopathy and could be a new target for future therapies.
Similar content being viewed by others
References
Albrecht, J., and Dolinska, M. (2001). Glutamine as a pathogenic factor in hepatic encephalopathy. J. Neurosci. Res. 65:1–5.
Balata, S., Damink, S.W., Ferguson, K., Marshall, I., Hayes, P.C., Deutz, N.E., Williams, R., Wardlaw, J., and Jalan, R. (2003). Induced hyperammonemia alters neuropsychology, brain MR spectroscopy and magnetization transfer in cirrhosis. Hepatology 37:931–939.
Corvera, S., and Garcia-Sainz, J.A. (1983). Hormonal stimulation of mitochondrial glutaminase. Effects of vasopressin, angiotensin II, adrenaline and glucagons. Biochem. J. 210:957–960.
Curthoys, N.P., and Watford, M. (1995). Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 15:133–159.
Hashimoto, N., Ashida, H., Kotoura, Y., Nishioka, A., Nishiwaki, M., and Utsunomiya, J. (1993). Analysis of hepatic encephalopathy after distal splenorenal shunt—PTP image and pancreatic hormone kinetics. Hepatogastroenterology 40:360–364.
Hawkins, R.A., Jessy, J., Mans, A.M., Chedid, A., and DeJoseph, M.R. (1994). Neomycin reduces the intestinal production of ammonia from glutamine. Adv. Exp. Med. Biol. 368:125–134.
Jalan, R., and Kapoor, D. (2004). Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin. Sci. (Lond.) 106:467–474.
James, L.A., Lunn, P.G., and Elia, M. (1988). Glutamine metabolism in the gastrointestinal tract of the rat assessed by the relative activity of gutaminase (EC 3.5.1.2) and glutamine synthetase (EC 6.3.1.2). Br. J. Nutr. 79:365–372.
James, L.A., Lunn, P.G., Middleton, S., and Elia, M. (1998). Distribution of glutaminase and glutamine synthetase activities in the human gastrointestinal tract. Clin Sci. 94:313–319.
Jayakumar, A.R., Rama Rao, K.V., Schousboe, A., and Norenberg, M.D. (2004). Glutamine-induced free radical production in cultured astrocytes. Glia 46:296–301.
Jover, M., Díaz, D., Collantes-de-Terán, L., Córpas, R., Fontiveros, E., Parrado, J., Bautista, J.D., and Romero-Gómez, M. (2005). Glutaminase activity is implicated in the pathogenesis of hyperammonemia in porto-caval shunted rats. J. Hepatol. 42(Suppl. 1):168A.
Kosenko, E., Llansola, M., Montoliu, C., Monfort, P., Rodrigo, R., Hernandez-Viadel, M., Erceg, S., Sanchez-Perez, A.M., and Felipo, V. (2003). Glutamine synthetase activity and glutamine content in brain: Modulation by NMDA receptors and nitric oxide. Neurochem. Int. 43:493–499.
McCauley, R., Kong, S.E., Heel, K., and Hall, J.C. (1999). The role of glutaminase in the small intestine. Int. J. Biochem. Cell Biol. 31:405–413.
Nance, F.C., and Kline, D.G. (1971). Eck's fistula encephalopathy in germ-free dogs. Ann. Surg. 174:856–861.
Norenberg, M.D. (1977). A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab. Invest. 36:618–627.
Norenberg, M.D., Rama Rao, K.V., and Jayakumar, A.R. (2004). Ammonia neurotoxicity and the mitochondrial permeability transition. J. Bioenerg. Biomembr. 36:303–307.
Olde Damink, S.W., Jalan, R., Redhead, D.N., Hayes, P.C., Deutz, N.E., and Soeters, P.B. (2002). Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 36:1163–1171.
Oppong, K.N., Al-Mardini, H., Thick, M., and Record, C.O. (1997). Oral glutamine challenge in cirrhotics pre- and post-liver transplantation: A psychometric and analysed EEG study. Hepatology 26:870–876.
Rama Rao, K.V., Jayakumar, A.R., and Norenberg, M.D. (2003). Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem. Int. 43:517–523.
Romero-Gómez, M., Bautista, J.D., Grande, L., Ramos Guerrero, R.M., and Sanchez Munoz, D. (2004a). New concepts in the physiopathology of hepatic encephalopathy and therapeutic prospects. Gastroenterol. Hepatol. 27(Suppl. 1):40–48.
Romero-Gomez, M., Grande, L., and Camacho, I. (2004b). Prognostic value of altered oral glutamine challenge in patients with minimal hepatic encephalopathy. Hepatology 39:939–943.
Romero-Gómez, M., Grande, L., Camacho, I., Benitez, S., Irles, J.A., and Castro, M. (2002). Altered response to oral glutamine challenge as prognostic factor for overt episodes in patients with minimal hepatic encephalopathy. J. Hepatol. 85:871–877.
Romero-Gómez, M., Hoyas, E., Viloria, M.M., Jover, M., Córpas, R., Collantes-de-Terán, L., Camacho, I., Cruz, M., and Bautista, J.D. (2005). Oral glutamine challenge response is regulated by portal hypertension and systemic inflammatory response. J. Hepatol. 42(Suppl. 1):182A.
Romero-Gomez, M., Ramos-Guerrero, R., Grande, L., de Teran, L.C., Corpas, R., Camacho, I., and Bautista, J.D. (2004c). Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J. Hepatol. 41:49–54.
Sherlock, S. (1987). Chronic portal-systemic encephalopathy: Update 1987. Gut 28:1043–1048.
Taylor, L., Liu, X., Newsome, W., Shapiro, R.A., Srinivasan, M., and Curthoys, N.P. (2001). Isolation and characterization of the promoter region of the rat kidney-type glutaminase gene. Biochim. Biophys. Acta 1518:132–136.
Warren, K.S., and Newton, W.L. (1959). Portal and peripheral blood ammonia concentrations in germ-free and conventional guinea pigs. Am. J. Physiol. 197:717–720.
Weber, F.J.L., and Veach, G.L. (1979). The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterology 77:235–240.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero-Gómez, M. Role of Phosphate-Activated Glutaminase in the Pathogenesis of Hepatic Encephalopathy. Metab Brain Dis 20, 319–325 (2005). https://doi.org/10.1007/s11011-005-7913-5
Issue Date:
DOI: https://doi.org/10.1007/s11011-005-7913-5