Abstract
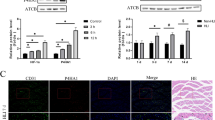
Angiogenesis is crucial for blood flow recovery and ischemic tissue repair of peripheral artery disease (PAD). Exploration of new mechanisms underlying angiogenesis will shed light on the treatment of PAD. Ubiquitin-fold modifier 1 (UFM1), a newly identified ubiquitin-like molecule, has been discovered to be involved in various pathophysiological processes. However, the role of UFM1 in the pathogenesis of PAD, especially in endothelial angiogenesis remains obscure, and we aimed to clarify this issue in this study. We initially found UFM1 was significantly upregulated in gastrocnemius muscles of PAD patients and hind limb ischemia mice. And UFM1 was mainly colocalized with endothelial cells in ischemic muscle tissues. Further, elevated expression of UFM1 was observed in hypoxic endothelial cells. Subsequent genetic inhibition of UFM1 dramatically enhanced migration, invasion, adhesion, and tube formation of endothelial cells under hypoxia. Mechanistically, UFM1 reduced the stability of hypoxia-inducible factor-1α (HIF-1α) and promoted the von Hippel–Lindau-mediated K48-linked ubiquitin–proteasome degradation of HIF-1α, which in turn decreased angiogenic factor VEGFA expression and suppressed VEGFA related signaling pathway. Consistently, overexpression of UFM1 inhibited the angiogenesis of endothelial cells under hypoxic conditions, whereas overexpression of HIF-1α reversed this effect. Collectively, our data reveal that UFM1 inhibits the angiogenesis of endothelial cells under hypoxia through promoting ubiquitin–proteasome degradation of HIF-1α, suggesting UFM1 might serve as a potential therapeutic target for PAD.










Similar content being viewed by others
Data availability
All data published in the paper will be available upon reasonable request.
References
Hackler ER, Hamburg NM, White SK (2021) Racial and ethnic disparities in peripheral artery disease. Circ Res 128(12):1913–1926. https://doi.org/10.1161/CIRCRESAHA.121.318243
Song P, Rudan D, Zhu Y, Fowkes F, Rahimi K, Fowkes F, Rudan I (2019) Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 7(8):e1020–e1030. https://doi.org/10.1016/S2214-109X(19)30255-4
Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G, Min J, Torii S, Poojary P, Anand SS, Bax JJ, Yusuf S, Virmani R, Narula J (2018) Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol 72(18):2152–2163. https://doi.org/10.1016/j.jacc.2018.08.002
Annex BH (2013) Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10(7):387–396. https://doi.org/10.1038/nrcardio.2013.70
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146(6):873–887. https://doi.org/10.1016/j.cell.2011.08.039
Han J, Luo L, Marcelina O, Kasim V, Wu S (2022) Therapeutic angiogenesis-based strategy for peripheral artery disease. Theranostics 12(11):5015–5033. https://doi.org/10.7150/thno.74785
Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P (2018) Endothelial cell metabolism. Physiol Rev 98(1):3–58. https://doi.org/10.1152/physrev.00001.2017
Herbert SP, Stainier DY (2011) Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12(9):551–564. https://doi.org/10.1038/nrm3176
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6(4):389–395. https://doi.org/10.1038/74651
Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9(6):677–684. https://doi.org/10.1038/nm0603-677
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WJ (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516):464–468. https://doi.org/10.1126/science.1059817
Kaelin WJ (2008) The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8(11):865–873. https://doi.org/10.1038/nrc2502
Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5(5):343–354. https://doi.org/10.1038/nrm1366
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292(5516):468–472. https://doi.org/10.1126/science.1059796
Komatsu M, Chiba T, Tatsumi K, Iemura S, Tanida I, Okazaki N, Ueno T, Kominami E, Natsume T, Tanaka K (2004) A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J 23(9):1977–1986. https://doi.org/10.1038/sj.emboj.7600205
Sasakawa H, Sakata E, Yamaguchi Y, Komatsu M, Tatsumi K, Kominami E, Tanaka K, Kato K (2006) Solution structure and dynamics of Ufm1, a ubiquitin-fold modifier 1. Biochem Biophys Res Commun 343(1):21–26. https://doi.org/10.1016/j.bbrc.2006.02.107
Banerjee S, Kumar M, Wiener R (2020) Decrypting UFMylation: how proteins are modified with UFM1. Biomolecules. https://doi.org/10.3390/biom10101442
Gerakis Y, Quintero M, Li H, Hetz C (2019) The UFMylation system in proteostasis and beyond. Trends Cell Biol 29(12):974–986. https://doi.org/10.1016/j.tcb.2019.09.005
Lu H, Yang Y, Allister EM, Wijesekara N, Wheeler MB (2008) The identification of potential factors associated with the development of type 2 diabetes: a quantitative proteomics approach. Mol Cell Proteomics 7(8):1434–1451. https://doi.org/10.1074/mcp.M700478-MCP200
Lemaire K, Moura RF, Granvik M, Igoillo-Esteve M, Hohmeier HE, Hendrickx N, Newgard CB, Waelkens E, Cnop M, Schuit F (2011) Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS ONE 6(4):e18517. https://doi.org/10.1371/journal.pone.0018517
Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE (2006) Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291(3):H1411–H1420. https://doi.org/10.1152/ajpheart.01378.2005
Li J, Yue G, Ma W, Zhang A, Zou J, Cai Y, Tang X, Wang J, Liu J, Li H, Su H (2018) Ufm1-specific ligase Ufl1 regulates endoplasmic reticulum homeostasis and protects against heart failure. Circ Heart Fail 11(10):e4917. https://doi.org/10.1161/CIRCHEARTFAILURE.118.004917
Cai Y, Zhu G, Liu S, Pan Z, Quintero M, Poole CJ, Lu C, Zhu H, Islam B, Riggelen JV, Browning D, Liu K, Blumberg R, Singh N, Li H (2019) Indispensable role of the Ubiquitin-fold modifier 1-specific E3 ligase in maintaining intestinal homeostasis and controlling gut inflammation. Cell Discov 5:7. https://doi.org/10.1038/s41421-018-0070-x
Yang R, Wang H, Kang B, Chen B, Shi Y, Yang S, Sun L, Liu Y, Xiao W, Zhang T, Yang J, Zhang Y, Zhu M, Xu P, Chang Y, Jia Y, Huang Y (2019) CDK5RAP3, a UFL1 substrate adaptor, is crucial for liver development. Development. https://doi.org/10.1242/dev.169235
Watson CM, Crinnion LA, Gleghorn L, Newman WG, Ramesar R, Beighton P, Wallis GA (2015) Identification of a mutation in the ubiquitin-fold modifier 1-specific peptidase 2 gene, UFSP2, in an extended South African family with Beukes hip dysplasia. S Afr Med J 105(7):558–563. https://doi.org/10.7196/SAMJnew.7917
Mignon-Ravix C, Milh M, Kaiser CS, Daniel J, Riccardi F, Cacciagli P, Nagara M, Busa T, Liebau E, Villard L (2018) Abnormal function of the UBA5 protein in a case of early developmental and epileptic encephalopathy with suppression-burst. Hum Mutat 39(7):934–938. https://doi.org/10.1002/humu.23534
Nahorski MS, Maddirevula S, Ishimura R, Alsahli S, Brady AF, Begemann A, Mizushima T, Guzmán-Vega FJ, Obata M, Ichimura Y, Alsaif HS, Anazi S, Ibrahim N, Abdulwahab F, Hashem M, Monies D, Abouelhoda M, Meyer BF, Alfadhel M, Eyaid W, Zweier M, Steindl K, Rauch A, Arold ST, Woods CG, Komatsu M, Alkuraya FS (2018) Biallelic UFM1 and UFC1 mutations expand the essential role of ufmylation in brain development. Brain 141(7):1934–1945. https://doi.org/10.1093/brain/awy135
Lin JX, Xie XS, Weng XF, Qiu SL, Yoon C, Lian NZ, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Yang YH, Huang CM, Zheng CH, Li P (2019) UFM1 suppresses invasive activities of gastric cancer cells by attenuating the expression of PDK1 through PI3K/AKT signaling. J Exp Clin Cancer Res 38(1):410. https://doi.org/10.1186/s13046-019-1416-4
Yoo HM, Kang SH, Kim JY, Lee JE, Seong MW, Lee SW, Ka SH, Sou YS, Komatsu M, Tanaka K, Lee ST, Noh DY, Baek SH, Jeon YJ, Chung CH (2014) Modification of ASC1 by UFM1 is crucial for ERα transactivation and breast cancer development. Mol Cell 56(2):261–274. https://doi.org/10.1016/j.molcel.2014.08.007
Zhou J, Ma X, Xu L, Liang Q, Mao J, Liu J, Wang M, Yuan J, Cong YS (2021) Genomic profiling of the UFMylation family genes identifies UFSP2 as a potential tumour suppressor in colon cancer. Clin Transl Med 11(12):e642. https://doi.org/10.1002/ctm2.642
Pang Q, Xiong J, Hu XL, He JP, Liu HF, Zhang GY, Li YY, Chen FL (2015) UFM1 Protects macrophages from oxLDL-induced foam cell formation through a liver X receptor α dependent pathway. J Atheroscler Thromb 22(11):1124–1140. https://doi.org/10.5551/jat.28829
Hu X, Pang Q, Shen Q, Liu H, He J, Wang J, Xiong J, Zhang H, Chen F (2014) Ubiquitin-fold modifier 1 inhibits apoptosis by suppressing the endoplasmic reticulum stress response in Raw264.7 cells. Int J Mol Med 33(6):1539–1546. https://doi.org/10.3892/ijmm.2014.1728
Li YY, Zhang GY, He JP, Zhang DD, Kong XX, Yuan HM, Chen FL (2017) Ufm1 inhibits LPS-induced endothelial cell inflammatory responses through the NF-κB signaling pathway. Int J Mol Med 39(5):1119–1126. https://doi.org/10.3892/ijmm.2017.2947
Niiyama H, Huang NF, Rollins MD, Cooke JP (2009) Murine model of hindlimb ischemia. J Vis Exp. https://doi.org/10.3791/1035
Kocherova I, Bryja A, Mozdziak P, Angelova VA, Dyszkiewicz-Konwińska M, Piotrowska-Kempisty H, Antosik P, Bukowska D, Bruska M, Iżycki D, Zabel M, Nowicki M, Kempisty B (2019) Human umbilical vein endothelial cells (HUVECs) co-culture with osteogenic cells: from molecular communication to engineering prevascularised bone grafts. J Clin Med. https://doi.org/10.3390/jcm8101602
Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176(6):1248–1264. https://doi.org/10.1016/j.cell.2019.01.021
Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR (2016) VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal 10(4):347–354. https://doi.org/10.1007/s12079-016-0352-8
Semenza GL (2014) Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76:39–56. https://doi.org/10.1146/annurev-physiol-021113-170322
Salceda S, Caro J (1997) Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272(36):22642–22647. https://doi.org/10.1074/jbc.272.36.22642
van Wijk SJ, Fulda S, Dikic I, Heilemann M (2019) Visualizing ubiquitination in mammalian cells. EMBO Rep. https://doi.org/10.15252/embr.201846520
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399(6733):271–275. https://doi.org/10.1038/20459
Witting KF, Mulder M (2021) Highly specialized ubiquitin-like modifications: shedding light into the UFM1 enigma. Biomolecules. https://doi.org/10.3390/biom11020255
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG (2007) Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45:S5–S67. https://doi.org/10.1016/j.jvs.2006.12.037
Yang J, Zhou Y, Xie S, Wang J, Li Z, Chen L, Mao M, Chen C, Huang A, Chen Y, Zhang X, Khan N, Wang L, Zhou J (2021) Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J Exp Clin Cancer Res 40(1):206. https://doi.org/10.1186/s13046-021-02012-7
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307. https://doi.org/10.1038/nature10144
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17(11):1359–1370. https://doi.org/10.1038/nm.2537
Holmes DI, Zachary I (2005) The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 6(2):209. https://doi.org/10.1186/gb-2005-6-2-209
Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E (2005) Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol 206(3):291–304. https://doi.org/10.1002/path.1778
Semenza GL (2003) Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54:17–28. https://doi.org/10.1146/annurev.med.54.101601.152418
Zhou J, Ma X, He X, Chen B, Yuan J, Jin Z, Li L, Wang Z, Xiao Q, Cai Y, Zou Y, Cong YS (2023) Dysregulation of PD-L1 by UFMylation imparts tumor immune evasion and identified as a potential therapeutic target. Proc Natl Acad Sci USA 120(11):e2079235176. https://doi.org/10.1073/pnas.2215732120
Hu Z, Wang X, Li D, Cao L, Cui H, Xu G (2021) UFBP1, a key component in ufmylation, enhances drug sensitivity by promoting proteasomal degradation of oxidative stress-response transcription factor Nrf2. Oncogene 40(3):647–662. https://doi.org/10.1038/s41388-020-01551-1
Luo H, Jiao QB, Shen CB, Gong WY, Yuan JH, Liu YY, Chen Z, Liu J, Xu XL, Cong YS, Zhang XW (2023) UFMylation of HRD1 regulates endoplasmic reticulum homeostasis. FASEB J 37(11):e23221. https://doi.org/10.1096/fj.202300004RRRR
Tao Y, Yin S, Liu Y, Li C, Chen Y, Han D, Huang J, Xu S, Zou Z, Yu Y (2023) UFL1 promotes antiviral immune response by maintaining STING stability independent of UFMylation. Cell Death Differ 30(1):16–26. https://doi.org/10.1038/s41418-022-01041-9
Liu J, Guan D, Dong M, Yang J, Wei H, Liang Q, Song L, Xu L, Bai J, Liu C, Mao J, Zhang Q, Zhou J, Wu X, Wang M, Cong YS (2020) UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat Cell Biol 22(9):1056–1063. https://doi.org/10.1038/s41556-020-0559-z
Du SC, Zhu L, Wang YX, Liu J, Zhang D, Chen YL, Peng Q, Liu W, Liu B (2019) SENP1-mediated deSUMOylation of USP28 regulated HIF-1α accumulation and activation during hypoxia response. Cancer Cell Int 19:4. https://doi.org/10.1186/s12935-018-0722-9
Yang Z, Huang Y, Zhu L, Yang K, Liang K, Tan J, Yu B (2021) SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1α and reactive oxygen species. Cell Death Dis 12(1):77. https://doi.org/10.1038/s41419-020-03372-2
Kwon SJ, Song JJ, Lee YJ (2005) Signal pathway of hypoxia-inducible factor-1alpha phosphorylation and its interaction with von Hippel–Lindau tumor suppressor protein during ischemia in MiaPaCa-2 pancreatic cancer cells. Clin Cancer Res 11(21):7607–7613. https://doi.org/10.1158/1078-0432.CCR-05-0981
Geng H, Harvey CT, Pittsenbarger J, Liu Q, Beer TM, Xue C, Qian DZ (2011) HDAC4 protein regulates HIF1α protein lysine acetylation and cancer cell response to hypoxia. J Biol Chem 286(44):38095–38102. https://doi.org/10.1074/jbc.M111.257055
Acknowledgements
We would like to acknowledge Professor Huaidong Song from the Core Laboratory in Medical Center of Clinical Research of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of Medicine. The schematic illustration was drawn with Figdraw.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number 82000258 and 81670735), Natural Science Foundation of Shanghai (Grant Number 22ZR1436500). Shanghai Ninth People’s Hospital Foundation, Shanghai JiaoTong University School of Medicine (Grant Number JYLJ201921, JYHJB04 and JYZP003).
Author information
Authors and Affiliations
Contributions
Yu Jing: Conceptualization, Software, Investigation, Resources, Writing—original draft, Writing—review & editing, Visualization. Kuanping Ye: Methodology, Software, Investigation, Data curation, Writing—review & editing. Guangya Zhang: Methodology, Validation, Funding acquisition. Jing Zhu: Validation, Data curation. Ziming Mao: Validation, Formal analysis. Qianru Zhang: Formal analysis. Fengling Chen: Conceptualization, Supervision, Project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All animal experiments were approved by the Ethics Committee of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, Y., Ye, K., Zhang, G. et al. UFM1 inhibits hypoxia-induced angiogenesis via promoting proteasome degradation of HIF-1α. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-05013-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-05013-0