Abstract
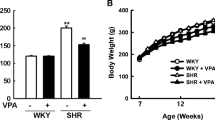
Central TRH, a neuropeptide, is involved in cardiovascular regulation. We demonstrated that the overexpression of diencephalic TRH (dTRH) in SHR rats can be prevented by antisense treatment, normalizing blood pressure (BP). Valproate (VPA) is an inhibitor of histone deacetylases (HDAC) which modulates gene expression through epigenetic modifications such as DNA methylation. Aims: Study the role of HDAC inhibition in the regulation of dTRH gene expression and its effect on the pathogenesis of hypertension. Main methods: We treated 7-weeks-old male and female SHR and WKY rats with VPA for 10 weeks and evaluated BP, dTRH mRNA and methylation gene status. Key findings: VPA attenuated the elevated BP and dTRH mRNA expression characteristic of SHR. Indeed, we found a significant 62% reduction in dTRH mRNA expression in the SHR + VPA group compared to control SHR. The decrease TRH mRNA expression induced by VPA was confirmed “in vitro” in a primary neuron culture using trichostatin A. With methylation specific PCR we demonstrated a significant increase in TRH promoter DNA methylation level in SHR + VPA group compared to control SHR. After 2 weeks of treatment interruption, rats were mated. Although they did not receive any treatment, the offspring born from VPA-treated SHR parents showed similar changes in BP, dTRH expression and methylation status, implying a transgenerational inheritance. Our findings suggest that dTRH modulation by epigenetics mechanism affects BP and could be inherited by the next generation in SHR rats.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Lechan RM, Jackson IMD (1982) Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary*. Endocrinology 111:55–65. https://doi.org/10.1210/endo-111-1-55
Wittmann G, Füzesi T, Liposits Z et al (2009) Distribution and axonal projections of neurons coexpressing thyrotropin-releasing hormone and urocortin 3 in the rat brain. J Comp Neurol 517:825–840. https://doi.org/10.1002/cne.22180
Lechan RM, Fekete C (2006) The TRH neuron: a hypothalamic integrator of energy metabolism. Progress in brain research. Elsevier, Amsterdam, pp 209–235
García SI, Porto PI, Alvarez AL et al (1997) Central overexpression of the TRH precursor gene induces hypertension in rats: antisense reversal. Hypertension 30:759–766. https://doi.org/10.1161/01.HYP.30.3.759
Landa MS, García SI, Schuman ML et al (2007) Knocking down the diencephalic thyrotropin-releasing hormone precursor gene normalizes obesity-induced hypertension in the rat. Am J Physiol-Endocrinol Metab 292:E1388–E1394. https://doi.org/10.1152/ajpendo.00234.2006
Burgueño AL, Landa MS, Schuman ML et al (2007) Association between diencephalic thyroliberin and arterial blood pressure in agouti-yellow and ob/ob mice may be mediated by leptin. Metabolism 56:1439–1443. https://doi.org/10.1016/j.metabol.2007.06.008
Landa MS (2007) SiRNA-mediated silencing of the diencephalic thyrotropin-releasing hormone precursor gene decreases the arterial blood pressure in the obese agouti mice. Front Biosci 12:3431. https://doi.org/10.2741/2324
Landa MS, García SI, Schuman ML et al (2020) Cardiovascular and body weight regulation changes in transgenic mice overexpressing thyrotropin-releasing hormone (TRH). J Physiol Biochem 76:599–608. https://doi.org/10.1007/s13105-020-00765-x
García SI, Alvarez AL, Porto PI et al (2001) Antisense inhibition of thyrotropin-releasing hormone reduces arterial blood pressure in spontaneously hypertensive rats. Hypertension 37:365–370. https://doi.org/10.1161/01.HYP.37.2.365
Li Y, Chen X, Lu C (2021) The interplay between DNA and histone methylation: molecular mechanisms and disease implications. EMBO Rep. https://doi.org/10.15252/embr.202051803
Zardo G, Fazi F, Travaglini L, Nervi C (2005) Dynamic and reversibility of heterochromatic gene silencing in human disease. Cell Res 15:679–690. https://doi.org/10.1038/sj.cr.7290337
Dong E, Chen Y, Gavin DP et al (2010) Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics 5:730–735. https://doi.org/10.4161/epi.5.8.13053
Aizawa S, Yamamuro Y (2015) Valproate administration to mice increases hippocampal p21 expression by altering genomic DNA methylation. NeuroReport 26:915–920. https://doi.org/10.1097/WNR.0000000000000448
Barciszewska A-M, Belter A, Gawrońska I et al (2022) Cross-reactivity between histone demethylase inhibitor valproic acid and DNA methylation in glioblastoma cell lines. Front Oncol 12:1033035. https://doi.org/10.3389/fonc.2022.1033035
Vila-Pueyo M, Cuenca-León E, Queirós AC et al (2023) Genome-wide DNA methylation analysis in an antimigraine-treated preclinical model of cortical spreading depolarization. Cephalalgia 43:033310242211463. https://doi.org/10.1177/03331024221146317
Cardinale JP, Sriramula S, Pariaut R et al (2010) HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 56:437–444. https://doi.org/10.1161/HYPERTENSIONAHA.110.154567
Sasaki S, Nakata T, Kawasaki S et al (1990) Chronic central GABAergic stimulation attenuates hypothalamic hyperactivity and development of spontaneous hypertension in rats. J Cardiovasc Pharmacol 15:706–713. https://doi.org/10.1097/00005344-199005000-00004
Chomzynski P (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159. https://doi.org/10.1006/abio.1987.9999
Peres Diaz LS, Schuman ML, Aisicovich M et al (2020) Short-term doxorubicin cardiotoxic effects: involvement of cardiac thyrotropin releasing hormone system. Life Sci 261:118346. https://doi.org/10.1016/j.lfs.2020.118346
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2−∆∆CT method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3:71–85
Kawasaki ES (1990) Sample preparation from blood, cells, and other fluids. PCR protocols. Elsevier, Amsterdam, pp 146–152
Li L-C, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427–1431. https://doi.org/10.1093/bioinformatics/18.11.1427
Gemma C, Sookoian S, Dieuzeide G et al (2010) Methylation of TFAM gene promoter in peripheral white blood cells is associated with insulin resistance in adolescents. Mol Genet Metab 100:83–87. https://doi.org/10.1016/j.ymgme.2010.02.004
Lopez Verrilli MA, Pirola CJ, Pascual MM et al (2009) Angiotensin-(1–7) through AT2 receptors mediates tyrosine hydroxylase degradation via the ubiquitin-proteasome pathway. J Neurochem 109:326–335. https://doi.org/10.1111/j.1471-4159.2009.05912.x
Hashimoto R, Hough C, Nakazawa T et al (2002) Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem 80:589–597. https://doi.org/10.1046/j.0022-3042.2001.00728.x
Nillni EA (2010) Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol 31:134–156. https://doi.org/10.1016/j.yfrne.2010.01.001
Díaz-Gallardo MY, Cote-Vélez A, Charli JL, Joseph-Bravo P (2010) A rapid interference between glucocorticoids and cAMP-activated signalling in hypothalamic neurones prevents binding of phosphorylated cAMP response element binding protein and glucocorticoid receptor at the CRE-like and composite GRE sites of thyrotrophin-releasing hormone gene promoter. J Neuroendocrinol 22:282–293. https://doi.org/10.1111/j.1365-2826.2010.01966.x
Lee H-A, Kang S-H, Kim M et al (2018) Histone deacetylase inhibition ameliorates hypertension and hyperglycemia in a model of Cushing’s syndrome. Am J Physiol-Endocrinol Metab 314:E39–E52. https://doi.org/10.1152/ajpendo.00267.2017
Choi J, Park S, Kwon TK et al (2017) Role of the histone deacetylase inhibitor valproic acid in high-fat diet-induced hypertension via inhibition of HDAC1/angiotensin II axis. Int J Obes 41:1702–1709. https://doi.org/10.1038/ijo.2017.166
Pekary AE, Sattin A, Meyerhoff JL, Chilingar M (2004) Valproate modulates TRH receptor, TRH and TRH-like peptide levels in rat brain. Peptides 25:647–658. https://doi.org/10.1016/j.peptides.2004.01.016
Kameshima S, Okada M, Yamawaki H (2016) Expression and localization of calmodulin-related proteins in brain, heart and kidney from spontaneously hypertensive rats. Biochem Biophys Res Commun 469:654–658. https://doi.org/10.1016/j.bbrc.2015.12.048
Lee RS, Pirooznia M, Guintivano J et al (2015) Search for common targets of lithium and valproic acid identifies novel epigenetic effects of lithium on the rat leptin receptor gene. Transl Psychiatry 5:e600–e600. https://doi.org/10.1038/tp.2015.90
Gu S, Tian Y, Chlenski A et al (2012) Valproic acid shows a potent antitumor effect with alteration of DNA methylation in neuroblastoma. Anticancer Drugs 23:1054–1066. https://doi.org/10.1097/CAD.0b013e32835739dd
Pedro Ferreira J, Pitt B, Zannad F (2021) Histone deacetylase inhibitors for cardiovascular conditions and healthy longevity. Lancet Healthy Longevity 2:e371–e379. https://doi.org/10.1016/S2666-7568(21)00061-1
Xin Y, Sun X, Ren L et al (2023) Maternal preconceptional inflammation transgenerationally alters metabolic and behavioral phenotypes in offspring. Life Sci 321:121577. https://doi.org/10.1016/j.lfs.2023.121577
Skinner MK (2014) Environmental stress and epigenetic transgenerational inheritance. BMC Med 12:153. https://doi.org/10.1186/s12916-014-0153-y
Master JS, Zimanyi MA, Yin KV et al (2014) Transgenerational left ventricular hypertrophy and hypertension in offspring after uteroplacental insufficiency in male rats. Clin Exp Pharmacol Physiol 41:884–890. https://doi.org/10.1111/1440-1681.12303
de Rooij SR, Painter RC, Phillips DI et al (2011) Self-reported depression and anxiety after prenatal famine exposure: mediation by cardio-metabolic pathology? J Devel Orig Health Dis 2:136–143. https://doi.org/10.1017/S2040174411000055
Dunn GA, Bale TL (2011) Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152:2228–2236. https://doi.org/10.1210/en.2010-1461
Anway MD, Cupp AS, Uzumcu M, Skinner MK (2005) Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469. https://doi.org/10.1126/science.1108190
Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK (2010) Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 5:e13100. https://doi.org/10.1371/journal.pone.0013100
Zheng X, Li Z, Wang G et al (2021) Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov 7:101. https://doi.org/10.1038/s41421-021-00343-5
Jia H, Morris CD, Williams RM et al (2015) HDAC inhibition imparts beneficial transgenerational effects in Huntington’s disease mice via altered DNA and histone methylation. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1415195112
Wu JN, Berecek KH (1993) Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension 22:139–146. https://doi.org/10.1161/01.HYP.22.2.139
Acknowledgements
The authors thank Noelia Gonzales Mansilla (Institute of Medical Research A. Lanari-IDIM, University of Buenos Aires-National Scientific and Technical Research Council (CONICET), Ciudad Autónoma de Buenos Aires, Argentina) for the technical assistance.
Funding
This work was supported by Grants PICT 2010-2581, PICT 2010-0441 (Agencia Nacional de Promoción Cientifica y Tecnologica), PIP 11220090100636 (Consejo Nacional de Investigaciones Científicas y Tecnicas) and PIP 11220200103056CO (Consejo Nacional de Investigaciones Científicas y Tecnicas).
Author information
Authors and Affiliations
Contributions
MSL, MLS, LSPD, MA, MMG, SIG analyzed data; MSL, SIG and CJP interpreted results of experiments; MSL, MLS, prepared figures; MSL, SIG and CJP drafted manuscript; MSL, MLS, SIG and CJP edited and revised manuscript and approved final version of manuscript; MSL, MLS, LSPD, MA and MMG performed experiments; CJP conception and design of research.
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Landa, M.S., Schuman, M.L., Aisicovich, M. et al. Valproate decreases transgenerationally blood pressure by affecting thyrotropin-releasing hormone promoter DNA methylation and gene expression in spontaneously hypertensive rat. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-05001-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-05001-4