Abstract
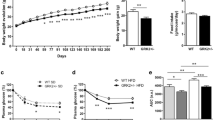
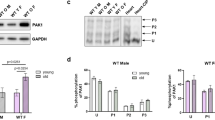
While P21-activated kinase-1 (PAK1) has been extensively studied in relation to cardiovascular health and glucose metabolism, its roles within adipose tissue and cardiometabolic diseases are less understood. In this study, we explored the effects of PAK1 deletion on energy balance, adipose tissue homeostasis, and cardiac function utilizing a whole-body PAK1 knockout (PAK1−/−) mouse model. Our findings revealed that body weight differences between PAK1−/− and WT mice emerged at 9 weeks of age, with further increases observed at 12 weeks. Furthermore, PAK1−/− mice displayed increased fat mass and decreased lean mass at 12 weeks, indicating a shift towards adiposity. In conjunction with the increased body weight, PAK1−/− mice had increased food intake and reduced energy expenditure. At a mechanistic level, PAK1 deletion boosted the expression of lipogenic markers while diminishing thermogenic markers expression in adipose tissues, contributing to reduced energy expenditure and the overall obesogenic phenotype. Moreover, our findings highlighted a significant impact on cardiac function following PAK1 deletion, including alterations in calcium kinetics and compromised systolic and lusitropy functions. In summary, our study emphasizes the significant role of PAK1 in weight regulation and cardiac function, enriching our comprehension of heart health and metabolism. These findings could potentially facilitate the identification of novel therapeutic targets in cardiometabolic diseases.







Similar content being viewed by others
Data availability
Data generated for this study are available on request to the corresponding author.
Abbreviations
- PAK1:
-
P21-activated kinase-1
- PAK1− / − :
-
PAK1 global knock out mouse
- gWAT:
-
Gonadal white adipose tissue
- scWAT:
-
Subcutaneous white adipose tissue
- iBAT:
-
Interscapular brown adipose tissue
- NMVMs:
-
Neonatal mouse ventricular myocytes
- UCP1:
-
Uncoupling protein 1
- Dio2:
-
Type 2 iodothyronine deiodinase
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator
- PRDM16:
-
PR domain containing 16
- LPL:
-
Lipoprotein lipase
- CPT1b:
-
Carnitine palmitoyl transferase 1b
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- Ca2+ :
-
Intracellular calcium
- PP2a:
-
Protein phosphatase 2a
- PP1:
-
Protein phosphatase 1
References
Lei M, Robinson MA, Harrison SC (2005) The active conformation of the PAK1 kinase domain. Structure 13:769–778. https://doi.org/10.1016/j.str.2005.03.007
Strochlic TI, Viaud J, Rennefahrt UE, Anastassiadis T, Peterson JR (2010) Phosphoinositides are essential coactivators for p21-activated kinase 1. Mol Cell 40:493–500. https://doi.org/10.1016/j.molcel.2010.10.015
Liu W, Zi M, Tsui H, Chowdhury SK, Zeef L, Meng QJ, Travis M, Prehar S, Berry A, Hanley NA, Neyses L, Xiao RP, Oceandy D, Ke Y, Solaro RJ, Cartwright EJ, Lei M, Wang X (2013) A novel immunomodulator, FTY-720 reverses existing cardiac hypertrophy and fibrosis from pressure overload by targeting NFAT (nuclear factor of activated T-cells) signaling and periostin. Circ Heart Fail 6:833–844. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000123
Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, Neyses L, Solaro RJ, Ke Y, Cartwright EJ, Lei M, Wang X (2011) Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation 124:2702–2715. https://doi.org/10.1161/CIRCULATIONAHA.111.048785
Monasky MM, Taglieri DM, Patel BG, Chernoff J, Wolska BM, Ke Y, Solaro RJ (2012) p21-activated kinase improves cardiac contractility during ischemia-reperfusion concomitant with changes in troponin-T and myosin light chain 2 phosphorylation. Am J Physiol Heart Circ Physiol 302:H224–H230. https://doi.org/10.1152/ajpheart.00612.2011
Taglieri DM, Monasky MM, Knezevic I, Sheehan KA, Lei M, Wang X, Chernoff J, Wolska BM, Ke Y, Solaro RJ (2011) Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J Mol Cell Cardiol 51:988–996. https://doi.org/10.1016/j.yjmcc.2011.09.016
Wang Y, Tsui H, Ke Y, Shi Y, Li Y, Davies L, Cartwright EJ, Venetucci L, Zhang H, Terrar DA, Huang CL, Solaro RJ, Wang X, Lei M (2014) Pak1 is required to maintain ventricular Ca(2)(+) homeostasis and electrophysiological stability through SERCA2a regulation in mice. Circ Arrhythm Electrophysiol 7:938–948. https://doi.org/10.1161/CIRCEP.113.001198
Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, Stringer SE, Ke Y, Shaheen M, Wang T, Chacko S, Wang X, Solaro RJ, Fath-Ordoubadi F, Cartwright EJ, Lei M (2011) Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol 301:H1487–H1495. https://doi.org/10.1152/ajpheart.01003.2010
Liu W, Zi M, Chi H, Jin J, Prehar S, Neyses L, Cartwright EJ, Flavell RA, Davis RJ, Wang X (2011) Deprivation of MKK7 in cardiomyocytes provokes heart failure in mice when exposed to pressure overload. J Mol Cell Cardiol 50:702–711. https://doi.org/10.1016/j.yjmcc.2011.01.013
Tunduguru R, Chiu TT, Ramalingam L, Elmendorf JS, Klip A, Thurmond DC (2014) Signaling of the p21-activated kinase (PAK1) coordinates insulin-stimulated actin remodeling and glucose uptake in skeletal muscle cells. Biochem Pharmacol 92:380–388. https://doi.org/10.1016/j.bcp.2014.08.033
Tunduguru R, Thurmond DC (2017) Promoting glucose transporter-4 vesicle trafficking along cytoskeletal tracks: PAK-ing them out. Front Endocrinol (Lausanne) 8:329. https://doi.org/10.3389/fendo.2017.00329
Merz KE, Tunduguru R, Ahn M, Salunkhe VA, Veluthakal R, Hwang J, Bhattacharya S, McCown EM, Garcia PA, Zhou C, Oh E, Yoder SM, Elmendorf JS, Thurmond DC (2022) Changes in skeletal muscle PAK1 levels regulate tissue crosstalk to impact whole body glucose homeostasis. Front Endocrinol (Lausanne) 13:821849. https://doi.org/10.3389/fendo.2022.821849
Sun J, Khalid S, Rozakis-Adcock M, Fantus IG, Jin T (2009) P-21-activated protein kinase-1 functions as a linker between insulin and Wnt signaling pathways in the intestine. Oncogene 28:3132–3144. https://doi.org/10.1038/onc.2009.167
Lim GE, Xu M, Sun J, Jin T, Brubaker PL (2009) The rho guanosine 5’-triphosphatase, cell division cycle 42, is required for insulin-induced actin remodeling and glucagon-like peptide-1 secretion in the intestinal endocrine L cell. Endocrinology 150:5249–5261. https://doi.org/10.1210/en.2009-0508
Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC (2011) Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem 286:41359–41367. https://doi.org/10.1074/jbc.M111.291500
Ahn M, Yoder SM, Wang Z, Oh E, Ramalingam L, Tunduguru R, Thurmond DC (2016) The p21-activated kinase (PAK1) is involved in diet-induced beta cell mass expansion and survival in mice and human islets. Diabetologia 59:2145–2155. https://doi.org/10.1007/s00125-016-4042-0
Sylow L, Jensen TE, Kleinert M, Hojlund K, Kiens B, Wojtaszewski J, Prats C, Schjerling P, Richter EA (2013) Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 62:1865–1875. https://doi.org/10.2337/db12-1148
Batra A, Warren CM, Ke Y, McCann M, Halas M, Capote AE, Liew CW, Solaro RJ, Rosas PC (2021) Deletion of P21-activated kinase-1 induces age-dependent increased visceral adiposity and cardiac dysfunction in female mice. Mol Cell Biochem 476:1337–1349. https://doi.org/10.1007/s11010-020-03993-3
Dammann K, Khare V, Lang M, Claudel T, Harpain F, Granofszky N, Evstatiev R, Williams JM, Pritchard DM, Watson A, Gasche C (2015) PAK1 modulates a PPARgamma/NF-kappaB cascade in intestinal inflammation. Biochim Biophys Acta 1853:2349–2360. https://doi.org/10.1016/j.bbamcr.2015.05.031
Hansson B, Moren B, Fryklund C, Vliex L, Wasserstrom S, Albinsson S, Berger K, Stenkula KG (2019) Adipose cell size changes are associated with a drastic actin remodeling. Sci Rep 9:12941. https://doi.org/10.1038/s41598-019-49418-0
DeSantiago J, Bare DJ, Xiao L, Ke Y, Solaro RJ, Banach K (2014) p21-Activated kinase1 (Pak1) is a negative regulator of NADPH-oxidase 2 in ventricular myocytes. J Mol Cell Cardiol 67:77–85. https://doi.org/10.1016/j.yjmcc.2013.12.017
Wu R, Park J, Qian Y, Shi Z, Hu R, Yuan Y, Xiong S, Wang Z, Yan G, Ong SG, Song Q, Song Z, Mahmoud AM, Xu P, He C, Arpke RW, Kyba M, Shu G, Jiang Q, Jiang Y (2023) Genetically prolonged beige fat in male mice confers long-lasting metabolic health. Nat Commun 14:2731. https://doi.org/10.1038/s41467-023-38471-z
Fritz JD, Swartz DR, Greaser ML (1989) Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal Biochem 180:205–210
Park J, Shin S, Liu L, Jahan I, Ong SG, Xu P, Berry DC, Jiang Y (2021) Progenitor-like characteristics in a subgroup of UCP1+ cells within white adipose tissue. Dev Cell 56(985–999):e4. https://doi.org/10.1016/j.devcel.2021.02.018
Solis C, Russell B (2019) CapZ integrates several signaling pathways in response to mechanical stiffness. J Gen Physiol 151:660–669. https://doi.org/10.1085/jgp.201812199
Dittloff KT, Spanghero E, Solis C, Banach K, Russell B (2022) Transthyretin deposition alters cardiomyocyte sarcomeric architecture, calcium transients, and contractile force. Physiol Rep 10:e15207. https://doi.org/10.14814/phy2.15207
Psaras Y, Margara F, Cicconet M, Sparrow AJ, Repetti GG, Schmid M, Steeples V, Wilcox JAL, Bueno-Orovio A, Redwood CS, Watkins HC, Robinson P, Rodriguez B, Seidman JG, Seidman CE, Toepfer CN (2021) CalTrack: high-throughput automated calcium transient analysis in cardiomyocytes. Circ Res 129:326–341. https://doi.org/10.1161/CIRCRESAHA.121.318868
Linkert M, Rueden CT, Allan C, Burel JM, Moore W, Patterson A, Loranger B, Moore J, Neves C, Macdonald D, Tarkowska A, Sticco C, Hill E, Rossner M, Eliceiri KW, Swedlow JR (2010) Metadata matters: access to image data in the real world. J Cell Biol 189:777–782. https://doi.org/10.1083/jcb.201004104
Capote AE, Batra A, Warren CM, Chowdhury SAK, Wolska BM, Solaro RJ, Rosas PC (2021) B-arrestin-2 signaling is important to preserve cardiac function during aging. Front Physiol 12:696852. https://doi.org/10.3389/fphys.2021.696852
Woods SC, D’Alessio DA (2008) Central control of body weight and appetite. J Clin Endocrinol Metab 93:S37-50. https://doi.org/10.1210/jc.2008-1630
Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A (2005) PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24:6465–6481. https://doi.org/10.1038/sj.onc.1208802
Chakrabarti P, Kandror KV (2009) FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem 284:13296–13300. https://doi.org/10.1074/jbc.C800241200
Wu J, Cohen P, Spiegelman BM (2013) Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 27:234–250. https://doi.org/10.1101/gad.211649.112
Kong D, Dagon Y, Campbell JN, Guo Y, Yang Z, Yi X, Aryal P, Wellenstein K, Kahn BB, Sabatini BL, Lowell BB (2016) A postsynaptic AMPK–>p21-activated kinase pathway drives fasting-induced synaptic plasticity in AgRP neurons. Neuron 91:25–33. https://doi.org/10.1016/j.neuron.2016.05.025
Grigoryan M, Kedees MH, Charron MJ, Guz Y, Teitelman G (2012) Regulation of mouse intestinal L cell progenitors proliferation by the glucagon family of peptides. Endocrinology 153:3076–3088. https://doi.org/10.1210/en.2012-1120
Braun M, Ramracheya R, Rorsman P (2012) Autocrine regulation of insulin secretion. Diabetes Obes Metab 14(Suppl 3):143–151. https://doi.org/10.1111/j.1463-1326.2012.01642.x
Chung JH, Kim DH, Kim YS, Son BS, Kim D, Hwang C, Shin D, Noh SG, Han JH, Kim DK, Kim JH, Koo JS, Chung HY, Yoon SH (2018) Upregulation of P21-activated kinase 1 (PAK1)/CREB axis in squamous non-small cell lung carcinoma. Cell Physiol Biochem 50:304–316. https://doi.org/10.1159/000494007
Pandey SC, Roy A, Zhang H, Xu T (2004) Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci 24:5022–5030. https://doi.org/10.1523/JNEUROSCI.5557-03.2004
Ahn M, Oh E, McCown EM, Wang X, Veluthakal R, Thurmond DC (2021) A requirement for PAK1 to support mitochondrial function and maintain cellular redox balance via electron transport chain proteins to prevent beta-cell apoptosis. Metabolism 115:154431. https://doi.org/10.1016/j.metabol.2020.154431
Saldivar-Ceron HI, Villamar-Cruz O, Wells CM, Oguz I, Spaggiari F, Chernoff J, Patino-Lopez G, Huerta-Yepez S, Montecillo-Aguado M, Rivera-Pazos CM, Loza-Mejia MA, Vivar-Sierra A, Briseno-Diaz P, Zentella-Dehesa A, Leon-Del-Rio A, Lopez-Saavedra A, Padierna-Mota L, Ibarra-Sanchez MJ, Esparza-Lopez J, Hernandez-Rivas R, Arias-Romero LE (2021) p21-activated kinase 1 promotes breast tumorigenesis via phosphorylation and activation of the calcium/calmodulin-dependent protein kinase II. Front Cell Dev Biol 9:759259. https://doi.org/10.3389/fcell.2021.759259
DeSantiago J, Bare DJ, Varma D, Solaro RJ, Arora R, Banach K (2018) Loss of p21-activated kinase 1 (Pak1) promotes atrial arrhythmic activity. Heart Rhythm 15:1233–1241. https://doi.org/10.1016/j.hrthm.2018.03.041
Pereira CH, Bare DJ, Rosas PC, Dias FAL, Banach K (2023) The role of P21-activated kinase (Pak1) in sinus node function. J Mol Cell Cardiol 179:90–101. https://doi.org/10.1016/j.yjmcc.2023.04.004
Funding
This work was supported by NIH/NHLBI K01HL155241 and AHA CDA849387 grants to PCR, and R01 DK132398 to YJ.
Author information
Authors and Affiliations
Contributions
MM, CS, MMc, JP, KRC, SAKC and PCR performed experiments. MM, CS and PCR prepared and wrote the manuscript. MM and PCR were involved in experimental design, and data analysis. YJ was involved in manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (AVI 15315 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Munoz, M., Solis, C., McCann, M. et al. P21-activated kinase-1 signaling is required to preserve adipose tissue homeostasis and cardiac function. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04968-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04968-4