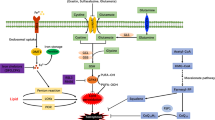
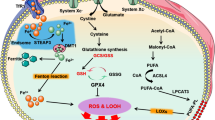
Abstract
Ferroptosis is a newly recognized type of regulated cell death that is characterized by the accumulation of iron and lipid peroxides in cells. Studies have shown that ferroptosis plays a significant role in the pathogenesis of various diseases, including cardiovascular diseases. In cardiovascular disease, ferroptosis is associated with ischemia–reperfusion injury, myocardial infarction, heart failure, and atherosclerosis. The molecular mechanisms underlying ferroptosis include the iron-dependent accumulation of lipid peroxidation products, glutathione depletion, and dysregulation of lipid metabolism, among others. This review aims to summarize the current knowledge of the molecular mechanisms of ferroptosis in cardiovascular disease and discuss the potential therapeutic strategies targeting ferroptosis as a treatment for cardiovascular disease.







Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Jiang XA-O, Stockwell BA-O, Conrad MA-O (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282. https://doi.org/10.1038/s41580-020-00324-8
Liang D, Minikes AM, Jiang X (2022) Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell 82(12):2215–2227. https://doi.org/10.1016/j.molcel.2022.03.022
Qiu Y, Cao Y, Cao W, Jia Y, Lu N (2020) The application of ferroptosis in diseases. Pharmacol Res 159:104919. https://doi.org/10.1016/j.phrs.2020.104919
Fang XA-O, Ardehali H, Min J, Wang FA-O (2023) The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20(1):7–23. https://doi.org/10.1038/s41569-022-00735-4
Guo Y, Lu C, Hu K, Cai C, Wang WA-O (2022) Ferroptosis in cardiovascular diseases: current status, challenges, and future perspectives. Biomolecules 12(3):390. https://doi.org/10.3390/biom12030390
Yang X, Kawasaki NK, Min J, Matsui T, Wang F (2022) Ferroptosis in heart failure. J Mol Cell Cardiol 173:141–153. https://doi.org/10.1016/j.yjmcc.2022.10.004
Bai T, Li M, Liu Y, Qiao Z, Wang Z (2020) Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med 160:92–102. https://doi.org/10.1016/j.freeradbiomed.2020.07.026
Zhao WA-O, Zhou YA-O, Xu TA-O, Wu QA-O (2021) Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid Med Cell Longev 2021:9929687. https://doi.org/10.1155/2021/9929687
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D et al (2019) Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 26(11):2284–2299. https://doi.org/10.1038/s41418-019-0299-4
Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, Tuomilehto J, Lip GYH, Penninger JM, Richardson DR et al (2021) Ferritinophagy and ferroptosis in the management of metabolic diseases. Endocrinol Metab 32(7):444–462. https://doi.org/10.1016/j.tem.2021.04.010
Hong M, Rong J, Tao X, Xu Y (2022) The emerging role of ferroptosis in cardiovascular diseases. Front Pharmacol 13:822083. https://doi.org/10.3389/fphar.2022.822083
Wu H, Wang F, Ta N, Zhang T, Gao W (2021) The multifaceted regulation of mitochondria in ferroptosis. Life (Basel) 11(3):222. https://doi.org/10.3390/life11030222
Wu S, Mao C, Kondiparthi L, Poyurovsky MV, Olszewski K, Gan BA-O (2022) A ferroptosis defense mechanism mediated by glycerol-3-phosphate dehydrogenase 2 in mitochondria. Proc Natl Acad Sci USA 119(26):e2121987119. https://doi.org/10.1073/pnas.2121987119
Jang S, Chapa-Dubocq XR, Tyurina YY, St Croix CM, Kapralov AA, Tyurin VA, Bayır H et al (2021) Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol 45:102021. https://doi.org/10.1016/j.redox.2021.102021
Haarhaus MA-O, Cianciolo G, Barbuto SA-O, La Manna G, Gasperoni L, Tripepi G, Plebani MA-O et al (2022) Alkaline phosphatase: an old friend as treatment target for cardiovascular and mineral bone disorders in chronic kidney disease. Nutrients 14(10):2124. https://doi.org/10.3390/nu14102124
Cardoso AC, Lam NT, Savla JJ, Nakada Y, Pereira AHM, Elnwasany A, Menendez-Montes I et al (2020) Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression. Nat Metab 2(2):167–178
Chan DC (2020) Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol 15:235–259. https://doi.org/10.1146/annurev-pathmechdis-012419-032711
Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, Li X et al (2021) Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell 184(11):2896-2910.e13. https://doi.org/10.1016/j.cell.2021.04.027
Read AD, Bentley RE, Archer SL, Dunham-Snary KJ (2021) Mitochondrial iron–sulfur clusters: structure, function, and an emerging role in vascular biology. Redox Biol 47:102164. https://doi.org/10.1016/j.redox.2021.102164
Fang XA-O, Wang HA-O, Han D, Xie E, Yang X, Wei J, Gu S et al (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA 116(7):2672–2680. https://doi.org/10.1073/pnas.1821022116
Kitakata H, Endo JA-O, Ikura H, Moriyama H, Shirakawa K, Katsumata Y, Sano M (2022) Therapeutic targets for dox-induced cardiomyopathy: role of apoptosis vs. ferroptosis. Int J Mol Sci 23(3):1414. https://doi.org/10.3390/ijms23031414
Jelinek A, Heyder L, Daude M, Plessner M, Krippner S, Grosse R, Diederich WE et al (2018) Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med 117:45–57. https://doi.org/10.1016/j.freeradbiomed.2018.01.019
Yang P, Luo X, Li J, Zhang T, Gao XA-O, Hua J, Li Y et al (2021) Ionizing radiation upregulates glutamine metabolism and induces cell death via accumulation of reactive oxygen species. Oxid Med Cell Longev 2021:5826932. https://doi.org/10.1155/2021/5826932
Fiore A, Zeitler L, Russier M, Groß A, Hiller MK, Parker JL, Stier L et al (2022) Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 82(5):920-932.e7. https://doi.org/10.1016/j.molcel.2022.02.007
Mishima EA-O, Ito J, Wu ZA-OX, Nakamura T, Wahida A, Doll SA-O, Tonnus WA-O et al (2022) A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608(7924):778–783. https://doi.org/10.1038/s41586-022-05022-3
Xie LW, Cai S, Zhao TS, Li M, Tian Y (2020) Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic Biol Med 161:175–186. https://doi.org/10.1016/j.freeradbiomed.2020.10.012
Yi H, Liu C, Shi J, Wang S, Zhang H, He Y, Tao J et al (2022) EGCG alleviates obesity-induced myocardial fibrosis in rats by enhancing expression of SCN5A. Front Cardiovasc Med 9:869279. https://doi.org/10.3389/fcvm.2022.869279
Hinman A, Holst CR, Latham JC, Bruegger JJ, Ulas G, McCusker KP, Amagata A et al (2018) Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS ONE 13(8):e0201369. https://doi.org/10.1371/journal.pone.0201369
Averill-Bates DA (2023) The antioxidant glutathione. Vitam Horm 121:109–141. https://doi.org/10.1016/bs.vh.2022.09.002
Bajic VP, Van Neste C, Obradovic M, Zafirovic SA-O, Radak D, Bajic VA-O, Essack MA-O et al (2019) Glutathione “redox homeostasis” and its relation to cardiovascular disease. Oxid Med Cell Longev 2019:5028181. https://doi.org/10.1155/2019/5028181
Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, Gao F et al (2020) Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 127(4):486–501. https://doi.org/10.1161/CIRCRESAHA.120.316509
Ta N, Qu C, Wu H, Zhang D, Sun T, Li Y, Wang J et al (2022) Mitochondrial outer membrane protein FUNDC2 promotes ferroptosis and contributes to doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA 119(36):e2117396119. https://doi.org/10.1073/pnas.2117396119
Ye Y, Chen A, Li L, Liang Q, Wang S, Dong Q, Fu M et al (2022) Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int 102(6):1259–1275. https://doi.org/10.1016/j.kint.2022.07.034
Xu S, Wu B, Zhong B, Lin L, Ding Y, Jin X, Huang Z et al (2021) Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/system xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 12(2):10924–10934. https://doi.org/10.1080/21655979.2021.1995994
Kumar P, Osahon OW, Sekhar RA-OX (2022) GlyNAC (glycine and N-acetylcysteine) supplementation in mice increases length of life by correcting glutathione deficiency, oxidative stress, mitochondrial dysfunction, abnormalities in mitophagy and nutrient sensing, and genomic damage. Nutrients 14(5):1114. https://doi.org/10.3390/nu14051114
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692. https://doi.org/10.1038/s41586-019-1705-2
Jia M, Qin D, Zhao C, Chai L, Yu Z, Wang W, Tong L et al (2020) Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol 21(7):727–735. https://doi.org/10.1038/s41590-020-0699-0
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191. https://doi.org/10.1038/ncb3064
Zhang Z, Tang J, Song J, Xie M, Liu Y, Dong Z, Liu X et al (2022) Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radic Biol Med 181:130–142. https://doi.org/10.1016/j.freeradbiomed.2022.01.020
Park TJ, Park JH, Lee GS, Lee JY, Shin JH, Kim MW, Kim YS et al (2019) Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis 10(11):835. https://doi.org/10.1038/s41419-019-2061-8
Wang YA-O, Yan SA-O, Liu XA-O, Deng FA-O, Wang P, Yang L, Hu LA-O et al (2022) PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ 29(10):1982–1995. https://doi.org/10.1038/s41418-022-00990-5
Feng Z, Qin Y, Huo F, Jian Z, Li X, Geng J, Li Y et al (2022) NMN recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation induced skin injury. Biochim Biophys Acta Mol Basis Dis 1868(1):166287. https://doi.org/10.1016/j.bbadis.2021.166287
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 152:175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M, Wang L et al (2022) Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol 289:115021. https://doi.org/10.1016/j.jep.2022.115021
Hong H, Lin X, Xu Y, Tong T, Zhang J, He H, Yang L et al (2022) Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic β-cells. Sci Total Environ 849:157819. https://doi.org/10.1016/j.scitotenv.2022.157819
Schwärzler J, Mayr L, Radlinger B, Grabherr F, Philipp M, Texler B, Grander C et al (2022) Adipocyte GPX4 protects against inflammation, hepatic insulin resistance and metabolic dysregulation. Int J Obes (Lond) 46(5):951–959. https://doi.org/10.1038/s41366-022-01064-9
Li Y, Wende AR, Nunthakungwan O, Huang Y, Hu E, Jin H, Boudina S, Dale Abel E, Jalili T (2012) Cytosolic, but not mitochondrial, oxidative stress is a likely contributor to cardiac hypertrophy resulting from cardiac specific GLUT4 deletion in mice. FEBS J 279(4):599–611. https://doi.org/10.1111/j.1742-4658.2011.08450.x
Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang CF, Li Y et al (2020) Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol Med Rep 22(1):175–184. https://doi.org/10.3892/mmr.2020.11114
Park E, Chung SW (2019) ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 10(11):822. https://doi.org/10.1038/s41419-019-2064-5
Ma J, Zhang H, Chen Y, Liu X, Tian J, Shen W (2022) The role of macrophage iron overload and ferroptosis in atherosclerosis. Biomolecules 12(11):1702. https://doi.org/10.3390/biom12111702
Sukhanov AA, Mamedov MD, Milanovsky GE, Salikhov KM, Semenov AY (2022) Changes in the electron transfer symmetry in the photosystem I reaction centers upon removal of iron–sulfur clusters. Biochem Mosc 87(10):1109–1118. https://doi.org/10.1134/S0006297922100042
Montoro-Huguet MA-O, Santolaria-Piedrafita S, Cañamares-Orbis P, García-Erce JA-O (2021) Iron deficiency in celiac disease: prevalence, health impact, and clinical management. Nutrients 13(10):3437. https://doi.org/10.3390/nu13103437
Wu X, Li Y, Zhang S, Zhou X (2021) Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11(7):3052–3059. https://doi.org/10.7150/thno.54113
Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q (2021) Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res 166:105466. https://doi.org/10.1016/j.phrs.2021.105466
Xie LA-O, Fefelova N, Pamarthi SH, Gwathmey JK (2022) Molecular mechanisms of ferroptosis and relevance to cardiovascular disease. Cells 11(17):2726. https://doi.org/10.3390/cells11172726
Stocki P, Szary J, Rasmussen CLM, Demydchuk M, Northall L, Logan DB, Gauhar A et al (2021) Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. FASEB J 35(2):e21172. https://doi.org/10.1096/fj.202001787R
Xiao Z, Kong B, Fang J, Qin T, Dai C, Shuai W, Huang H (2021) Ferrostatin-1 alleviates lipopolysaccharide-induced cardiac dysfunction. Bioengineered 12(2):9367–9376. https://doi.org/10.1080/21655979.2021.2001913
Du W, Wang J, Zhou L, Zhou J, Feng L, Dou C, Zhang Q et al (2023) Transferrin-targeted iridium nanoagglomerates with multi-enzyme activities for cerebral ischemia-reperfusion injury therapy. Acta Biomater 166:524–535. https://doi.org/10.1016/j.actbio.2023.04.025
Yanatori I, Yasui Y, Noguchi Y, Kishi F (2015) Inhibition of iron uptake by ferristatin II is exerted through internalization of DMT1 at the plasma membrane. Cell Biol Int 39(4):427–434. https://doi.org/10.1002/cbin.10403
Muscella AA-O, Stefàno E, Marsigliante S (2020) The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Heart Circ Physiol 319(1):H76–H88. https://doi.org/10.1152/ajpheart.00708.2019
Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, Tyurin VA et al (2017) PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171(3):628-641.e26. https://doi.org/10.1016/j.cell.2017.09.044
Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H, Walther M (2010) Molecular enzymology of lipoxygenases. Arch Biochem Biophys 503(2):161–174. https://doi.org/10.1016/j.abb.2010.08.016
Gao S, Zhou LA-O, Lu J, Fang Y, Wu H, Xu W, Pan Y et al (2022) Cepharanthine attenuates early brain injury after subarachnoid hemorrhage in mice via inhibiting 15-lipoxygenase-1-mediated microglia and endothelial cell ferroptosis. Oxid Med Cell Longev 2022:4295208
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BA-O (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113(34):E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Singh NK, Rao GN (2019) Emerging role of 12/15-lipoxygenase (ALOX15) in human pathologies. Prog Lipid Res 73:28–45. https://doi.org/10.1016/j.plipres.2018.11.001
He S, Li R, Peng Y, Wang Z, Huang J, Meng H, Min J et al (2022) ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke. J Cachexia Sarcopenia Muscle 13(3):1717–1730. https://doi.org/10.1002/jcsm.12953
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler MA-OX et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98. https://doi.org/10.1038/nchembio.2239
Pei Z, Liu Y, Liu S, Jin W, Luo Y, Sun M, Duan Y et al (2021) FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. Metabolism 122:154840. https://doi.org/10.1016/j.metabol.2021.154840
Zhou Y, Zhou H, Hua L, Hou C, Jia Q, Chen J, Zhang S et al (2021) Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic Biol Med 171:55–68. https://doi.org/10.1016/j.freeradbiomed.2021.05.009
Askari B, Kanter JE, Sherrid AM, Golej DL, Bender AT, Liu J, Hsueh WA, Beavo JA et al (2007) Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 56(4):1143–1152. https://doi.org/10.2337/db06-0267
Liu L, Kang XX (2022) ACSL4 is overexpressed in psoriasis and enhances inflammatory responses by activating ferroptosis. Biochem Biophys Res Commun 623:1–8. https://doi.org/10.1016/j.bbrc.2022.07.041
Quan J, Bode AM, Luo X (2021) ACSL family: the regulatory mechanisms and therapeutic implications in cancer. Eur J Pharmacol 909:174397. https://doi.org/10.1016/j.ejphar.2021.174397
Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye ZP, Peng XD et al (2022) PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol 24(1):88–98. https://doi.org/10.1038/s41556-021-00818-3
Yuan Y, Mei Z, Qu Z, Li G, Yu S, Liu Y, Liu K et al (2023) Exosomes secreted from cardiomyocytes suppress the sensitivity of tumor ferroptosis in ischemic heart failure. Signal Transduct Target Ther 8(1):121. https://doi.org/10.1038/s41392-023-01336-4
Chen YA-O, Li S, Yin M, Li Y, Chen C, Zhang J, Sun K et al (2023) Isorhapontigenin attenuates cardiac microvascular injury in diabetes via the inhibition of mitochondria-associated ferroptosis through PRDX2-MFN2-ACSL4 pathways. Diabetes 72(3):389–404. https://doi.org/10.2337/db22-0553
Chen XA-O, Kang RA-O, Kroemer GA-O, Tang DA-O (2021) Ferroptosis in infection, inflammation, and immunity. J Exp Med 218(6):e20210518. https://doi.org/10.1084/jem.20210518
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, Xu S et al (2020) The emerging role of ferroptosis in inflammation. Biomed Pharmacother 127:110108. https://doi.org/10.1016/j.biopha.2020.110108
Kar F, Yıldız F, Hacioglu C, Kar E, Donmez DB, Senturk H, Kanbak G (2023) LoxBlock-1 or curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in Ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. Tissue Cell 82:102114. https://doi.org/10.1016/j.tice.2023.102114
Kim Y, Clifton PA-OX (2018) Curcumin, cardiometabolic health and dementia. Int J Environ Res Public Health 15(10):2093. https://doi.org/10.3390/ijerph15102093
Abrahams S, Haylett WL, Johnson G, Carr JA, Bardien S (2019) Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: a review. Neuroscience 406:1–21. https://doi.org/10.1016/j.neuroscience.2019.02.020
Yu T, Dohl J, Wang L, Chen Y, Gasier HG, Deuster PA (2020) Curcumin ameliorates heat-induced injury through NADPH oxidase-dependent redox signaling and mitochondrial preservation in C2C12 myoblasts and mouse skeletal muscle. J Nutr 150(9):2257–2267. https://doi.org/10.1093/jn/nxaa201
Kim G, Piao C, Oh J, Lee M (2019) Combined delivery of curcumin and the heme oxygenase-1 gene using cholesterol-conjugated polyamidoamine for anti-inflammatory therapy in acute lung injury. Phytomedicine 56:165–174. https://doi.org/10.1016/j.phymed.2018.09.240
Chen Y, Li C, Duan S, Yuan X, Liang J, Hou S (2019) Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed Pharmacother 118:109195. https://doi.org/10.1016/j.biopha.2019.109195
Li X, Zhu R, Jiang H, Yin Q, Gu J, Chen J, Ji X et al (2022) Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm Sin B 12(5):2280–2299. https://doi.org/10.1016/j.apsb.2021.12.014
Pivari FA-O, Mingione AA-O, Piazzini G, Ceccarani CA-O, Ottaviano EA-O, Brasacchio C, Dei Cas MA-O et al (2022) Curcumin supplementation (Meriva(®)) modulates inflammation, lipid peroxidation and gut microbiota composition in chronic kidney disease. Nutrients 14(1):231. https://doi.org/10.3390/nu14010231
Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, Wang Q et al (2022) Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B 12(2):708–722. https://doi.org/10.1016/j.apsb.2021.10.005
Zhang Y, Wu Q, Liu J, Zhang Z, Ma X, Zhang Y, Zhu J et al (2022) Sulforaphane alleviates high fat diet-induced insulin resistance via AMPK/Nrf2/GPx4 axis. Biomed Pharmacother 152:113273. https://doi.org/10.1016/j.biopha.2022.113273
Ishida K, Kaji K, Sato S, Ogawa H, Takagi H, Takaya H, Kawaratani H et al (2021) Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem 89:108573. https://doi.org/10.1016/j.jnutbio.2020.108573
Li D, Shao R, Wang N, Zhou N, Du K, Shi J, Wang Y et al (2021) Sulforaphane activates a lysosome-dependent transcriptional program to mitigate oxidative stress. Autophagy 17(4):872–887. https://doi.org/10.1080/15548627.2020.1739442
Franke M, Bieber M, Kraft P, Weber ANR, Stoll G, Schuhmann MK (2021) The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav Immun 92:223–233. https://doi.org/10.1016/j.bbi.2020.12.009
Vashi R, Patel BA-O (2021) NRF2 in cardiovascular diseases: a ray of hope! J Cardiovasc Transl Res 14(3):573–586. https://doi.org/10.1007/s12265-020-10083-8
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107. https://doi.org/10.1016/j.redox.2019.101107
Baird L, Yamamoto M (2020) The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol 40(13):e00099-20. https://doi.org/10.1128/MCB.00099-20
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res 1865(5):721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Yuan Y, Zhai Y, Chen J, Xu X, Wang HA-O (2021) Kaempferol ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal ferroptosis by activating Nrf2/SLC7A11/GPX4 axis. Biomolecules 11(7):923. https://doi.org/10.3390/biom11070923
Dong H, Qiang Z, Chai D, Peng J, Xia Y, Hu R, Jiang H (2020) Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY) 12(13):12943–12959. https://doi.org/10.18632/aging.103378
Wang Y, Mandal AK, Son YO, Pratheeshkumar P, Wise JTF, Wang L, Zhang Z et al (2018) Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol Appl Pharmacol 353:23–30. https://doi.org/10.1016/j.taap.2018.06.003
Ghafouri-Fard SA-OX, Shoorei HA-O, Bahroudi Z, Hussen BA-O, Talebi SF, Taheri M, Ayatollahi SA (2022) Nrf2-related therapeutic effects of curcumin in different disorders. Biomolecules 12(1):82. https://doi.org/10.3390/biom12010082
Bonnefont-Rousselot D (2016) Resveratrol and cardiovascular diseases. Nutrients 8(5):250. https://doi.org/10.3390/nu8050250
Yang W, Wang Y, Zhang C, Huang Y, Yu J, Shi L, Zhang P et al (2022) Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Front Pharmacol 13:865689. https://doi.org/10.3389/fphar.2022.865689
Li Y, Xu B, Ren X, Wang L, Xu Y, Zhao Y, Yang C et al (2022) Inhibition of CISD2 promotes ferroptosis through ferritinophagy-mediated ferritin turnover and regulation of p62-Keap1-NRF2 pathway. Cell Mol Biol Lett 27(1):81. https://doi.org/10.1186/s11658-022-00383-z
Zhang Q, Liu J, Duan H, Li R, Peng W, Wu C (2021) Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res 34:43–63. https://doi.org/10.1016/j.jare.2021.06.023
Rehman S, Mahboob T, Kamali MF (2022) Correlation between serum malondialdehyde levels and prevalence of cardiovascular disease in haemodialysis treated patients. J Pak Med Assoc 72(8):1557–1580. https://doi.org/10.47391/JPMA.4402
Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A, Vučković AM et al (2020) Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol 28:101328. https://doi.org/10.1016/j.redox.2019.101328
Ouyang S, Li H, Lou L, Huang Q, Zhang Z, Mo J, Li M et al (2022) Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol 52:102317. https://doi.org/10.1016/j.redox.2022.102317
Agunbiade TA, Zaghlol RY, Barac A (2019) Heart failure in relation to anthracyclines and other chemotherapies. Methodist DeBakey Cardiovasc J 15(4):243–249. https://doi.org/10.14797/mdcj-15-4-243
Gao W, Wang X, Zhou Y, Wang X, Yu Y (2022) Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther 7(1):196. https://doi.org/10.1038/s41392-022-01046-3
Chen Z, Yan Y, Qi C, Liu J, Li L, Wang J (2021) The role of ferroptosis in cardiovascular disease and its therapeutic significance. Front Cardiovasc Med 8:733229. https://doi.org/10.3389/fcvm.2021.733229
Wang XD, Kang S (2021) Ferroptosis in myocardial infarction: not a marker but a maker. Open Biol 11(4):200367. https://doi.org/10.1098/rsob.200367
Cena H, Calder PC (2020) Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients 12(2):334. https://doi.org/10.3390/nu12020334
Funding
This work was supported by the Excellent Youth Project of Hunan Provincial Education Department under Grant No.22B0411.
Author information
Authors and Affiliations
Contributions
Qun Zeng: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, final approval of the version to be published. Tingting Jiang: Drafting the article or revising it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Q., Jiang, T. Molecular mechanisms of ferroptosis in cardiovascular disease. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04940-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04940-2