Abstract
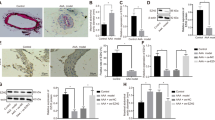
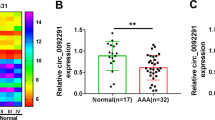
Ferroptosis of vascular smooth muscle cells (VSMCs) is related to the incidence of aortic dissection (AD). Long non-coding RNA (lncRNA) NORAD plays a crucial role in the progression of various diseases. The present study aimed to investigate the effects of NORAD on the ferroptosis of VSMCs and the molecular mechanisms. The expression of NORAD, HUR, and GPX4 was detected using quantitative real-time PCR (qPCR) or western blot. Ferroptosis was evaluated by detecting lactate dehydrogenase (LDH) activity, lipid reactive oxygen species (ROS), malonaldehyde (MDA) content, L-Glutathione (GSH) level, Fe2+ content, and ferroptosis-related protein levels. The molecular mechanism was assessed using RNA pull-down, RNA-binding protein immunoprecipitation (RIP), and luciferase reporter assay. The histology of aortic tissues was assessed using H&E, elastic Verhoeff–Van Gieson (EVG), and Masson staining assays. The data indicated that NORAD was downregulated in patients with AD and AngII-treated VSMCs. Overexpression of NORAD promoted VSMC growth and inhibited the ferroptosis induced by AngII. Mechanistically, NORAD interacted with HUR, which promoted GPX4 mRNA stability and elevated GPX4 levels. Knockdown of GPX4 abrogated the effects of NORAD on cell growth and ferroptosis of AngII-treated VSMCs. Moreover, METTL3 promoted m6A methylation of NORAD in an YTHDF2-dependent manner. In addition, NORAD attenuated AAD symptoms, incidence, histopathology, inflammation, and ferroptosis in AAD mice. In conclusion, METTL3-mediated NORAD inhibited ferroptosis of VSMCs via the HUR/GPX4 axis and decelerated AAD progression, suggesting that NORAD may be an AD therapeutic target.









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jun C, Fang B (2021) Current progress of fluoroquinolones-increased risk of aortic aneurysm and dissection. BMC Cardiovasc Disord 21(1):470
Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, Criqui MH (2014) Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart 9(1):171-180.e10
Gawinecka J, Schönrath F, von Eckardstein A (2017) Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly 147:w14489
Sayed A, Munir M, Bahbah EI (2021) Aortic dissection: a review of the pathophysiology, management and prospective advances. Curr Cardiol Rev 17(4):e230421186875
Sen I, Erben YM, Franco-Mesa C, DeMartino RR (2021) Epidemiology of aortic dissection. Semin Vasc Surg 34(1):10–17
Jassar AS, Sundt TM 3rd (2019) How should we manage type A aortic dissection? Gen Thorac Cardiovasc Surg 67(1):137–145
Clément M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, Taleb S, Jørgensen HF, Mallat Z (2019) Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler Thromb Vasc Biol 39(6):1149–1159
Bridges MC, Daulagala AC, Kourtidis A (2021) LNCcation: lncRNA localization and function. J Cell Biol 220(2):e202009045
Soghli N, Yousefi T, Abolghasemi M, Qujeq D (2021) NORAD, a critical long non-coding RNA in human cancers. Life Sci 264:118665
Munschauer M, Nguyen CT, Sirokman K, Hartigan CR, Hogstrom L, Engreitz JM, Ulirsch JC, Fulco CP, Subramanian V, Chen J, Schenone M, Guttman M, Carr SA, Lander ES (2018) The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature 561(7721):132–136
Bian W, Jing X, Yang Z, Shi Z, Chen R, Xu A, Wang N, Jiang J, Yang C, Zhang D, Li L, Wang H, Wang J, Sun Y, Zhang C (2020) Downregulation of LncRNA NORAD promotes Ox-LDL-induced vascular endothelial cell injury and atherosclerosis. Aging (Albany NY) 12(7):6385–6400
Shi L, Cong YZ, Wang ZJ (2021) LncRNA NORAD promotes thyroid carcinoma progression by targeting miR-451. Eur Rev Med Pharmacol Sci 25(20):6187–6195
Li S, Zhu X, Zhang N, Cao R, Zhao L, Li X, Zhang J, Yu J (2021) LncRNA NORAD engages in psoriasis by binding to miR-26a to regulate keratinocyte proliferation. Autoimmunity 54(3):129–137
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020) Ferroptosis: past, present and future. Cell Death Dis 11(2):88
Qiu Y, Cao Y, Cao W, Jia Y, Lu N (2020) The application of ferroptosis in diseases. Pharmacol Res 159:104919
Jin R, Yang R, Cui C, Zhang H, Cai J, Geng B, Chen Z (2022) Ferroptosis due to cystathionine γ lyase/hydrogen sulfide downregulation under high hydrostatic pressure exacerbates VSMC dysfunction. Front Cell Dev Biol 10:829316
Sampilvanjil A, Karasawa T, Yamada N, Komada T, Higashi T, Baatarjav C, Watanabe S, Kamata R, Ohno N, Takahashi M (2020) Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 318(3):H508–H518
Trachet B, Aslanidou L, Piersigilli A, Fraga-Silva RA, Sordet-Dessimoz J, Villanueva-Perez P, Stampanoni MFM, Stergiopulos N, Segers P (2017) Angiotensin II infusion into ApoE-/- mice: a model for aortic dissection rather than abdominal aortic aneurysm? Cardiovasc Res 113(10):1230–1242
Xu H, Du S, Fang B, Li C, Jia X, Zheng S, Wang S, Li Q, Su W, Wang N, Zheng F, Chen L, Zhang X, Gustafsson JÅ, Guan Y (2019) VSMC-specific EP4 deletion exacerbates angiotensin II-induced aortic dissection by increasing vascular inflammation and blood pressure. Proc Natl Acad Sci USA 116(17):8457–8462
Yang W, Wang Y, Zhang C, Huang Y, Yu J, Shi L, Zhang P, Yin Y, Li R, Tao K (2022) Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Front Pharmacol 13:865689
Yue L, Luo Y, Jiang L, Sekido Y, Toyokuni S (2022) PCBP2 knockdown promotes ferroptosis in malignant mesothelioma. Pathol Int 72(4):242–251
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31(2):107–125
Li N, Yi X, He Y, Huo B, Chen Y, Zhang Z, Wang Q, Li Y, Zhong X, Li R, Zhu XH, Fang Z, Wei X, Jiang DS (2022) Targeting ferroptosis as a novel approach to alleviate aortic dissection. Int J Biol Sci 18(10):4118–4134
Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q (2021) Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res 166:105466
Yao J, Chen X, Liu X, Li R, Zhou X, Qu Y (2021) Characterization of a ferroptosis and iron-metabolism related lncRNA signature in lung adenocarcinoma. Cancer Cell Int 21(1):340
Zhang B, Bao W, Zhang S, Chen B, Zhou X, Zhao J, Shi Z, Zhang T, Chen Z, Wang L, Zheng X, Chen G, Wang Y (2022) LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis 13(8):734
Ni T, Huang X, Pan S, Lu Z (2021) Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. J Cell Mol Med 25(21):9995–10007
Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C (2022) Lipid peroxidation and iron metabolism: two corner stones in the homeostasis control of ferroptosis. Int J Mol Sci 24(1):449
Ferrè F, Colantoni A, Helmer-Citterich M (2016) Revealing protein-lncRNA interaction. Brief Bioinform 17(1):106–116
Yu C, Xin W, Zhen J, Liu Y, Javed A, Wang R, Wan Q (2015) Human antigen R mediated post-transcriptional regulation of epithelial-mesenchymal transition related genes in diabetic nephropathy. J Diabetes 7(4):562–572
Grammatikakis I, Abdelmohsen K, Gorospe M (2017) Posttranslational control of HuR function. Wiley Interdiscip Rev RNA. https://doi.org/10.1002/wrna.1372
Schultz CW, Preet R, Dhir T, Dixon DA, Brody JR (2020) Understanding and targeting the disease-related RNA binding protein human antigen R (HuR). Wiley Interdiscip Rev RNA 11(3):e1581
Ding F, Lu L, Wu C, Pan X, Liu B, Zhang Y, Wang Y, Wu W, Yan B, Zhang Y, Yu XY, Li Y (2022) circHIPK3 prevents cardiac senescence by acting as a scaffold to recruit ubiquitin ligase to degrade HuR. Theranostics 12(17):7550–7566
Liu S, Jiang X, Cui X, Wang J, Liu S, Li H, Yang J, Zhang C, Zhang W (2021) Smooth muscle-specific HuR knockout induces defective autophagy and atherosclerosis. Cell Death Dis 12(4):385
Forcina GC, Dixon SJ (2019) GPX4 at the Crossroads of Lipid homeostasis and ferroptosis. Proteomics 19(18):e1800311
Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T (2017) Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol 403:143–170
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 152:175–185
Zhang Y, Xin L, Xiang M, Shang C, Wang Y, Wang Y, Cui X, Lu Y (2022) The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother 145:112423
Chen Y, Yi X, Huo B, He Y, Guo X, Zhang Z, Zhong X, Feng X, Fang ZM, Zhu XH, Wei X, Jiang DS (2022) BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol Res 177:106122
Trachet B, Fraga-Silva RA, Jacquet PA, Stergiopulos N, Segers P (2015) Incidence, severity, mortality, and confounding factors for dissecting AAA detection in angiotensin II-infused mice: a meta-analysis. Cardiovasc Res 108(1):159–170
Li G, Ma L, He S, Luo R, Wang B, Zhang W, Song Y, Liao Z, Ke W, Xiang Q, Feng X, Wu X, Zhang Y, Wang K, Yang C (2022) WTAP-mediated m6A modification of lncRNA NORAD promotes intervertebral disc degeneration. Nat Commun 13(1):1469
Yang J, Fang M, Yu C, Li Z, Wang Q, Li C, Wu J, Fan R (2023) Human aortic smooth muscle cell regulation by METTL3 via upregulation of m6A NOTCH1 modification and inhibition of NOTCH1. Ann Transl Med 11(10):350
Sun CY, Cao D, Du BB, Chen CW, Liu D (2022) The role of Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) as m6A readers in cancer. Int J Biol Sci 18(7):2744–2758
Wang Y, Wang Y, Gu J, Su T, Gu X, Feng Y (2022) The role of RNA m6A methylation in lipid metabolism. Front Endocrinol (Lausanne) 8(13):866116
Funding
This study was supported by the National Natural Science Foundation of China (No.81470576; No.81570440).
Author information
Authors and Affiliations
Contributions
SZ and JW conceived the study; JB and YL conducted the experiments; KZ and LQ analyzed the data; ML and SZ wrote the manuscript; all the authors read and approved the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that no conflicts of interest exist in this work.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Naval Medical University. Written informed consent was provided by all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, M., Zou, S., Wu, J. et al. METTL3-mediated m6A modification of NORAD inhibits the ferroptosis of vascular smooth muscle cells to attenuate the aortic dissection progression in an YTHDF2-dependent manner. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04930-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04930-4