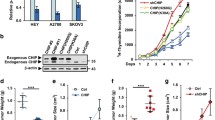
Abstract
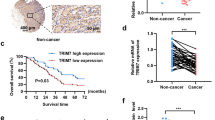
The E3 ubiquitin ligase Tripartite-motif 3 (TRIM3) is known to play a crucial role in tumor suppression in various tumors through different mechanisms. However, its function and mechanism in ovarian cancer have yet to be elucidated. Our study aims to investigate the expression of TRIM3 in ovarian cancer and evaluate its role in the development of the disease. Our findings revealed a significant decrease in TRIM3 mRNA and protein levels in ovarian cancer tissues and cells when compared to normal ovarian epithelial tissues and cells. Furthermore, we observed a negative correlation between the protein level of TRIM3 and the FIGO stage, as well as a positive correlation with the survival of ovarian cancer patients. Using gain and loss of function experiments, we demonstrated that TRIM3 can inhibit cell proliferation, migration and invasion of the ovarian cancer cells in vitro, as well as suppress tumor growth in vivo. Mechanistic studies showed that TRIM3 interacts with lactate dehydrogenase A, a key enzyme in the glycolytic pathway, through its B-box and coiled-coil domains and induces its ubiquitination and proteasomal degradation, leading to the inhibition of glycolytic ability in ovarian cancer cells. RNA-sequencing analysis revealed significant alterations in the phosphatidylinositol signaling pathways upon TRIM3 overexpression. Additionally, overexpression of TRIM3 inhibited the phosphorylation of AKT. In conclusion, our study demonstrated that TRIM3 exerts a tumor-suppressive effect in ovarian cancer, at least partially, by downregulating LDHA and inhibiting the AKT signaling pathway, and thus leading to the inhibition of glycolysis and limiting the growth of ovarian cancer cells.
Graphical abstract
Our study mainly investigated the role and mechanism of the E3 ubiquitin ligase Tripartite-motif 3 (TRIM3) in the progression of ovarian cancer. As a tumor suppressor, TRIM3 interact directly with LDHA and promotes the ubiquitination and degradation of LDHA, which plays a crucial role in the conversion of pyruvate and NADH to L-lactate and NAD in the final step of anaerobic glycolysis, thereby inhibiting glycolysis in ovarian cancer cells. Furthermore, overexpression of TRIM3 also reduces the phosphorylation of AKT independently of the interaction between TRIM3 and LDHA. In summary, our findings demonstrated that TRIM3 could inhibit ovarian cancer progression by downregulating LDHA and suppressing the AKT signaling pathway.








Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TRIM3:
-
The E3 ubiquitin ligase Tripartite-motif 3
- FIGO:
-
International federation of gynecology and obstetrics
- GEPIA:
-
Gene expression profiling interactive analysis
- TCGA:
-
The cancer genome atlas
- GTEx:
-
Genome-tissue expression
- ECAR:
-
Extracellular acidification rate
- PFK1:
-
Phosphofructokinase 1
- PFKP:
-
Phosphofructokinase 1 platelet type
- PFKM:
-
Phosphofructokinase 1 muscle type
- PFKL:
-
Phosphofructokinase 1 liver type
- GLUT1:
-
Glucose transporter protein
- LDHA:
-
Lactate dehydrogenase A
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- UPS:
-
Ubiquitin-proteasome system
- TBST:
-
Tris-buffered saline containing 0.1% Tween-20
- CHX:
-
Cycloheximide
References
Bhatnagar S, Gazin C, Chamberlain L, Ou J, Zhu X, Tushir JS, Virbasius CM, Lin L, Zhu LJ, Wajapeyee N, Green MR (2014) TRIM37 is a new histone H2A ubiquitin ligase and breast cancer oncoprotein. Nature 516:116–120. https://doi.org/10.1038/nature13955
Carthagena L, Bergamaschi A, Luna JM, David A, Uchil PD, Margottin-Goguet F, Mothes W, Hazan U, Transy C, Pancino G, Nisole S (2009) Human TRIM gene expression in response to interferons. PLoS ONE 4:e4894. https://doi.org/10.1371/journal.pone.0004894
Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KKC, Flores LG, Shao Y, Hazle JD, Yu D, Wei W, Sarbassov D, Hung MC, Nakayama KI, Lin HK (2012) The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 151:913–914. https://doi.org/10.1016/j.cell.2012.10.025
Chen G, Kong J, Tucker-Burden C, Anand M, Rong Y, Rahman F, Moreno CS, Van Meir EG, Hadjipanayis CG, Brat DJ (2014) Human Brat ortholog TRIM3 is a tumor suppressor that regulates asymmetric cell division in glioblastoma. Cancer Res 74:4536–4548. https://doi.org/10.1158/0008-5472.CAN-13-3703
Di Rienzo M, Romagnoli A, Antonioli M, Piacentini M, Fimia GM (2020) TRIM proteins in autophagy: selective sensors in cell damage and innate immune responses. Cell Death Differ 27:887–902. https://doi.org/10.1038/s41418-020-0495-2
Dunaway GA, Kasten TP (1987) Nature of the subunits of the 6-phosphofructo-1-kinase isoenzymes from rat tissues. Biochem J 242:667–671. https://doi.org/10.1042/bj2420667
Dunaway GA, Kasten TP, Sebo T, Trapp R (1988) Analysis of the phosphofructokinase subunits and isoenzymes in human tissues. Biochem J 251:677–683. https://doi.org/10.1042/bj2510677
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64:3892–3899. https://doi.org/10.1158/0008-5472.CAN-03-2904
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9:425–434. https://doi.org/10.1016/j.ccr.2006.04.023
Ganapathy-Kanniappan S, Geschwind JF (2013) Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152. https://doi.org/10.1186/1476-4598-12-152
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N (2001) Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15:1406–1418. https://doi.org/10.1101/gad.889901
Gyorffy B (2023) Discovery and ranking of the most robust prognostic biomarkers in serous ovarian cancer. Geroscience 45:1889–1898. https://doi.org/10.1007/s11357-023-00742-4
Gyorffy B, Benke Z, Lanczky A, Balazs B, Szallasi Z, Timar J, Schafer R (2012) RecurrenceOnline: an online analysis tool to determine breast cancer recurrence and hormone receptor status using microarray data. Breast Cancer Res Treat 132:1025–1034. https://doi.org/10.1007/s10549-011-1676-y
Hansen JM, Coleman RL, Sood AK (2016) Targeting the tumour microenvironment in ovarian cancer. Eur J Cancer 56:131–143. https://doi.org/10.1016/j.ejca.2015.12.016
Hatakeyama S (2011) TRIM proteins and cancer. Nat Rev Cancer 11:792–804. https://doi.org/10.1038/nrc3139
Hatakeyama S (2017) TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci 42:297–311. https://doi.org/10.1016/j.tibs.2017.01.002
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: warburg and beyond. Cell 134:703–707. https://doi.org/10.1016/j.cell.2008.08.021
Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, Oh D, Varma G, Hung YP, Ullas S, Lauring J, Seth P, Lundquist MR, Tolan DR, Grant AK, Needleman DJ, Asara JM, Cantley LC, Wulf GM (2016) Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell 164:433–446. https://doi.org/10.1016/j.cell.2015.12.042
Huang XQ, Zhang XF, Xia JH, Chao J, Pan QZ, Zhao JJ, Zhou ZQ, Chen CL, Tang Y, Weng DS, Zhang JH, Xia JC (2017) Tripartite motif-containing 3 (TRIM3) inhibits tumor growth and metastasis of liver cancer. Chin J Cancer 36:77. https://doi.org/10.1186/s40880-017-0240-5
Jaworska AM, Wlodarczyk NA, Mackiewicz A, Czerwinska P (2020) The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cells 38:165–173. https://doi.org/10.1002/stem.3109
Jessmon P, Boulanger T, Zhou W, Patwardhan P (2017) Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev Anticancer Ther 17:427–437. https://doi.org/10.1080/14737140.2017.1299575
Kahn A, Meienhofer MC, Cottreau D, Lagrange JL, Dreyfus JC (1979) Phosphofructokinase (PFK) isozymes in man. I. Studies of adult human tissues. Hum Genet 48:93–108. https://doi.org/10.1007/BF00273280
Kuroki L, Guntupalli SR (2020) Treatment of epithelial ovarian cancer. BMJ 371:m3773. https://doi.org/10.1136/bmj.m3773
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV (2010) Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107:2037–2042. https://doi.org/10.1073/pnas.0914433107
Lheureux S, Braunstein M, Oza AM (2019) Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin 69:280–304. https://doi.org/10.3322/caac.21559
Li Q, Birkbak NJ, Gyorffy B, Szallasi Z, Eklund AC (2011) Jetset: selecting the optimal microarray probe set to represent a gene. BMC Bioinformatics 12:474. https://doi.org/10.1186/1471-2105-12-474
Li WW, Nie Y, Yang Y, Ran Y, Luo WW, Xiong MG, Wang SY, Xu ZS, Wang YY (2020) Ubiquitination of TLR3 by TRIM3 signals its ESCRT-mediated trafficking to the endolysosomes for innate antiviral response. Proc Natl Acad Sci U S A 117:23707–23716. https://doi.org/10.1073/pnas.2002472117
Li X, Sodroski J (2008) The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol 82:11495–11502. https://doi.org/10.1128/JVI.01548-08
Li X, Song B, Xiang SH, Sodroski J (2007) Functional interplay between the B-box 2 and the B30.2(SPRY) domains of TRIM5alpha. Virology 366:234–244. https://doi.org/10.1016/j.virol.2007.04.022
Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ (2001) Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry 40:5542–5547. https://doi.org/10.1021/bi002426w
Massiah MA, Simmons BN, Short KM, Cox TC (2006) Solution structure of the RBCC/TRIM B-box1 domain of human MID1: B-box with a RING. J Mol Biol 358:532–545. https://doi.org/10.1016/j.jmb.2006.02.009
McNab FW, Rajsbaum R, Stoye JP, O′Garra A (2011) Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol 23:46–56. https://doi.org/10.1016/j.coi.2010.10.021
Micale L, Chaignat E, Fusco C, Reymond A, Merla G (2012) The tripartite motif: structure and function. Adv Exp Med Biol 770:11–25
Mor I, Cheung EC, Vousden KH (2011) Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol 76:211–216. https://doi.org/10.1101/sqb.2011.76.010868
Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274:1393–1418. https://doi.org/10.1111/j.1742-4658.2007.05686.x
Mukherjee S, Tucker-Burden C, Zhang C, Moberg K, Read R, Hadjipanayis C, Brat DJ (2016) Drosophila brat and human ortholog trim3 maintain stem cell equilibrium and suppress brain tumorigenesis by attenuating notch nuclear transport. Cancer Res 76:2443–2452. https://doi.org/10.1158/0008-5472.CAN-15-2299
Nakayama KI, Nakayama K (2006) Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6:369–381. https://doi.org/10.1038/nrc1881
Piao MY, Cao HL, He NN, Xu MQ, Dong WX, Wang WQ, Wang BM, Zhou B (2016) Potential role of TRIM3 as a novel tumour suppressor in colorectal cancer (CRC) development. Scand J Gastroenterol 51:572–582. https://doi.org/10.3109/00365521.2015.1124285
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A (2001) The tripartite motif family identifies cell compartments. EMBO J 20:2140–2151. https://doi.org/10.1093/emboj/20.9.2140
Robey RB, Hay N (2009) Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 19:25–31. https://doi.org/10.1016/j.semcancer.2008.11.010
Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G (2012) Knockdown of lactate dehydrogenase a suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J 279:3898–3910. https://doi.org/10.1111/j.1742-4658.2012.08748.x
Short KM, Cox TC (2006) Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem 281:8970–8980. https://doi.org/10.1074/jbc.M512755200
Song Y, Guo Q, Gao S, Hua K (2018) Tripartite motif-containing protein 3 plays a role of tumor inhibitor in cervical cancer. Biochem Biophys Res Commun 498:686–692. https://doi.org/10.1016/j.bbrc.2018.03.046
Takayama KI, Suzuki T, Tanaka T, Fujimura T, Takahashi S, Urano T, Ikeda K, Inoue S (2018) TRIM25 enhances cell growth and cell survival by modulating p53 signals via interaction with G3BP2 in prostate cancer. Oncogene 37:2165–2180. https://doi.org/10.1038/s41388-017-0095-x
Tocchini C, Ciosk R (2015) TRIM-NHL proteins in development and disease. Semin Cell Dev Biol 47–48:52–59. https://doi.org/10.1016/j.semcdb.2015.10.017
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68:284–296. https://doi.org/10.3322/caac.21456
Venuto S, Merla G (2019) E3 ubiquitin ligase TRIM proteins cell cycle and mitosis. Cells. https://doi.org/10.3390/cells8050510
Wei WS, Chen X, Guo LY, Li XD, Deng MH, Yuan GJ, He LY, Li YH, Zhang ZL, Jiang LJ, Chen RX, Ma XD, Wei S, Ma NF, Liu ZW, Luo JH, Zhou FJ, Xie D (2018) TRIM65 supports bladder urothelial carcinoma cell aggressiveness by promoting ANXA2 ubiquitination and degradation. Cancer Lett 435:10–22. https://doi.org/10.1016/j.canlet.2018.07.036
Weng ML, Chen WK, Chen XY, Lu H, Sun ZR, Yu Q, Sun PF, Xu YJ, Zhu MM, Jiang N, Zhang J, Zhang JP, Song YL, Ma D, Zhang XP, Miao CH (2020) Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1alpha pathway suppression. Nat Commun 11:1869. https://doi.org/10.1038/s41467-020-15795-8
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, Jiang B, Feng J, Li J, Gu Y (2019) PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med Rep 19:783–791. https://doi.org/10.3892/mmr.2018.9713
Xu K, Yin N, Peng M, Stamatiades EG, Shyu A, Li P, Zhang X, Do MH, Wang Z, Capistrano KJ, Chou C, Levine AG, Rudensky AY, Li MO (2021) Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 371:405–410. https://doi.org/10.1126/science.abb2683
Yang W, Lu Z (2015) Pyruvate kinase M2 at a glance. J Cell Sci 128:1655–1660. https://doi.org/10.1242/jcs.166629
Yao F, Zhao T, Zhong C, Zhu J, Zhao H (2013) LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol 34:25–31. https://doi.org/10.1007/s13277-012-0506-0
Zhang L, Afolabi LO, Wan X, Li Y, Chen L (2020) Emerging roles of tripartite motif-containing family proteins (TRIMs) in eliminating misfolded proteins. Front Cell Dev Biol 8:802. https://doi.org/10.3389/fcell.2020.00802
Zhang Y, Feng Y, Ji D, Wang Q, Qian W, Wang S, Zhang Z, Ji B, Zhang C, Sun Y, Fu Z (2018) TRIM27 functions as an oncogene by activating epithelial-mesenchymal transition and p-AKT in colorectal cancer. Int J Oncol 53:620–632. https://doi.org/10.3892/ijo.2018.4408
Zhou X, Chen R, Xie W, Ni Y, Liu J, Huang G (2014) Relationship between 18F-FDG accumulation and lactate dehydrogenase a expression in lung adenocarcinomas. J Nucl Med 55:1766–1771. https://doi.org/10.2967/jnumed.114.145490
Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, Gao WQ (2014) TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology 147:1043–1054. https://doi.org/10.1053/j.gastro.2014.07.021
Zhu J, Wu G, Ke Z, Cao L, Tang M, Li Z, Li Q, Zhou J, Tan Z, Song L, Li J (2019) Targeting TRIM3 deletion-induced tumor-associated lymphangiogenesis prohibits lymphatic metastasis in esophageal squamous cell carcinoma. Oncogene 38:2736–2749. https://doi.org/10.1038/s41388-018-0621-5
Funding
This study was financially supported by Jiangsu provincial key research and development program (Grant No: BE2020753, BE2019621), Jiangsu Provincial Medical Talent (Xuemei Jia), Nanjing Medical Science and Technique Development Foundation (Grant No: ZDX16015). Jiangsu Province Capability Improvement Project through Science, Technology and Education Jiangsu Provincial Medical Key Discipline (ZDXK202211).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. XJ and JX: Conceptualization, Supervision and Funding acquisition; YC and XC: Investigation, Methodology, Validation, writing-original draft; YS and XP: Methodology, Visualization and Formal Analysis; KH and ZG: Methodology, Resources; PX, LG and JZ: Resources and Data curation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
The authors declare that there are no conflicts of interest.
Consent for publication
Not applicable.
Ethics approval
The experimental protocol and method were approved by the Ethics Committee of Women′s Hospital of Nanjing Medical University (Nanjing Women and Children's Healthcare Hospital) and followed the Declaration of Helsinki (Approved number: 2022KY-176, approved on 2-10-2023). Informed consents were obtained from all participates. The in vivo experiments were approved by the ethics committee of Nanjing Medical University (Approved number: IACUC-2208015, approved on 8-18-2022).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cong, Y., Cui, X., Shi, Y. et al. Tripartite-motif 3 represses ovarian cancer progression by downregulating lactate dehydrogenase A and inhibiting AKT signaling. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-023-04920-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04920-y