Abstract
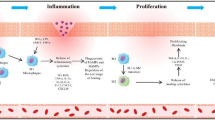
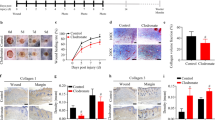
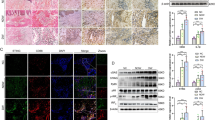
The alteration of inflammatory phenotype by macrophage polarization plays an important role in diabetic wound repair. Apigenin has been reported to be anti-inflammatory and promote tissue repair; however, whether it regulates macrophage polarization to participate in diabetic wound repair remains to be investigated. We found that apigenin promoted miR-21 expression in LPS-stimulated RAW264.7 cells, inhibited cellular M1-type factor TNF-α and IL-1β secretion and increased M2-type factor IL-10 and TGF-β secretion, and accelerated macrophage conversion from M1 type to M2 type, whereas this protective effect of apigenin was counteracted by a miR-21 inhibitor. Moreover, we established a macrophage-HUVECs cell in vitro co-culture system and found that apigenin accelerated the migration, proliferation, and VEGF secretion of HUVECs by promoting macrophage miR-21 expression. Further, mechanistic studies revealed that this was mediated by the TLR4/Myd88/NF-κB axis. In in vivo study, diabetic mice had significantly delayed wound healing compared to non-diabetic mice, accelerated wound healing in apigenin-treated diabetic mice, and decreased M1-type macrophages and increased M2-type macrophages in wound tissues.





Similar content being viewed by others
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Abbreviations
- LPS:
-
Lipopolysaccharide
- IFN-γ:
-
Interferon-γ
- IL-4:
-
Interleukin-4
- COX-2:
-
Cyclooxygenase-2
- HUVECs:
-
Human umbilical vein endothelial cells
References
Louiselle AE et al (2021) Macrophage polarization and diabetic wound healing. Transl Res 236:109–116
Aitcheson SM et al (2021) Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules 26(16):4917
Boniakowski AE et al (2017) Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol 199(1):17–24
Liu W et al (2020) Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther 11(1):259
Davis FM et al (2020) Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight. https://doi.org/10.1172/jci.insight.138443
Gan J et al (2019) Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials 219:119340
Polerà N et al (2019) Quercetin and its natural sources in wound healing management. Curr Med Chem 26(31):5825–5848
Lu X et al (2022) Dietary prenylated flavonoid xanthohumol alleviates oxidative damage and accelerates diabetic wound healing via Nrf2 activation. Food Chem Toxicol 160:112813
Park CH et al (2020) Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int J Mol Sci 21(13):4620
Zhou Q et al (2019) Anti-inflammatory effect of an apigenin-maillard reaction product in macrophages and macrophage-endothelial cocultures. Oxid Med Cell Longev 2019:9026456
Che DN et al (2019) Apigenin inhibits IL-31 cytokine in human mast cell and mouse skin tissues. Molecules 24(7):1290
Wu Y et al (2020) MicroRNA-21-3p accelerates diabetic wound healing in mice by downregulating SPRY1. Aging (Albany NY) 12(15):15436–15445
Lv Q et al (2020) Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm 17(5):1723–1733
Li Q et al (2019) Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int J Biochem Cell Biol 114:105570
Yao M et al (2021) Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci 264:118658
Tu F et al (2017) Angiogenic effects of apigenin on endothelial cells after hypoxia-reoxygenation via the caveolin-1 pathway. Int J Mol Med 40(6):1639–1648
Liu SC et al (2021) Adipose-derived stem cell induced-tissue repair or wound healing is mediated by the concomitant upregulation of miR-21 and miR-29b expression and activation of the AKT signaling pathway. Arch Biochem Biophys 705:108895
Peng X et al (2022) miR-146a promotes M2 macrophage polarization and accelerates diabetic wound healing by inhibiting the TLR4/NF-κB axis. J Mol Endocrinol 69(2):315–327
Portou MJ et al (2020) Hyperglycaemia and ischaemia impair wound healing via toll-like receptor 4 pathway activation in vitro and in an experimental murine model. Eur J Vasc Endovasc Surg 59(1):117–127
Acknowledgements
I would like to express my gratitude to all those helped me during the writing of this thesis. I acknowledge the help of my colleagues, Lijun Wu; they have offered me suggestion in academic studies.
Funding
This study was supported by Suzhou People’s Livelihood Science and Technology Project (SYS2020105); Construction of key clinical specialties for the Suzhou Municipal “Strengthening Health through Science and Education” Funding Project; Hospital Research Fund (SDFEYBS1805, SDFEYGJ2013, XKTJ-HRC20210015); Suzhou Science and Technology Development Project (SYS2020105, SKJY2021078 and 2022SS43); the Special Project of “Technological Innovation” Project of CNNC Medical Industry Co. Ltd (ZHYLZD2021002); Project of State Key Laboratory of Radiation Medicine and Protection, Soochow University, (No. GZK1202244); and the CNNC Elite Talent Program.
Author information
Authors and Affiliations
Contributions
KL and JJ: designed the experiments. KL and LW: performed the experimental LW and JJ: provided statistical analysis and figures for the manuscript. KL: wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11010_2023_4885_MOESM1_ESM.tif
Supplementary file1 (TIF 2342 kb)— The secretion of inflammatory factors in non-activated RAW264.7 cells. The RAW264.7 cells (M0 macrophages) were left untreated or treated with apigenin alone. A&B&C&D. The secretion levels of TNF-α, IL-1β, IL-10, and TGF-β in RAW264.7 cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, K., Wu, L. & Jiang, J. Apigenin accelerates wound healing in diabetic mice by promoting macrophage M2-type polarization via increasing miR-21 expression. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-023-04885-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04885-y