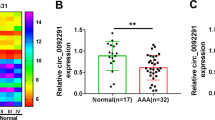
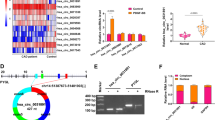
Abstract
Dihydroartemisinin (DHA) inhibits restenosis following balloon angioplasty. However, data on the mechanisms of DHA activity in restenosis remains scant. Here, we investigated the role of circRNAs in mediating the inhibitory activity of DHA in neointimal formation. We used total RNA sequencing data to profile the expression of mRNA, circRNA and small RNA in sham, vascular balloon injury (VBI) and DHA-treated groups. CCK8 and EdU assays were employed to analyze cell proliferation, while qRT-PCR and Western blot were used to analyze the RNA or protein expression. In addition, we used RNA immunoprecipitation and luciferase reporter assay to assess the binding of circHSPA4 with miR-19a-5p. RNA sequencing demonstrated that circHSPA4 was upregulated in VBI. Treatment with DHA effectively suppressed the upregulation of the circHSPA4. In addition, analysis of platelet-derived growth family factor bb (PDGFbb)-induced HA-VSMCs showed upregulation of circHSPA4, which was associated with cell proliferation and differentiation. CircHSPA4 was shown to induce dedifferentiation and proliferation of smooth muscle cells. PDGFBB-induced overexpression of CircHSPA4 in HA-VSMCs led to suppression of miR-19a-5p, a phenomenon that was reversed by DHA, in concentration-dependent fashion. In addition, miR-19a-5p reduced the dedifferentiation and proliferation of the smooth muscle cells. Our data demonstrated that CircHSPA4 regulates proliferation and differentiation of smooth muscle cells. DHA and miR-19a-5p modulates CircHSPA4 and can be used as coated drugs on balloon catheter to improve the success rate of vascular remodeling.






Similar content being viewed by others
Data availability
Data will be made available on request.
References
Golledge J, Drovandi A (2021) Evidence-based recommendations for medical management of peripheral artery disease. J Atheroscler Thromb 28(6):573–583
Cooke JP, Meng S (2020) Vascular regeneration in peripheral artery disease. Arterioscler Thromb Vasc Biol 40(7):1627–1634
Hussain MA, Al-Omran M, Creager MA, Anand SS, Verma S, Bhatt DL (2018) Antithrombotic therapy for peripheral artery disease: recent advances. J Am Coll Cardiol 71(21):2450–2467
Morcos R, Louka B, Tseng A, Misra S, McBane R, Esser H, Shamoun F (2018) The evolving treatment of peripheral arterial disease through guideline-directed recommendations. J Clin Med 7(1):9
Hess CN, Norgren L, Ansel GM, Capell WH, Fletcher JP, Fowkes FGR, Gottsater A, Hitos K, Jaff MR, Nordanstig J et al (2017) A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: a TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) Initiative. Circulation 135(25):2534–2555
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S et al (2017) 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 135(12):e726–e779
Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ et al (2015) Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 131(5):495–502
Bausback Y, Wittig T, Schmidt A, Zeller T, Bosiers M, Peeters P, Brucks S, Lottes AE, Scheinert D, Steiner S (2019) Drug-eluting stent versus drug-coated balloon revascularization in patients with femoropopliteal arterial disease. J Am Coll Cardiol 73(6):667–679
Tu Y (2016) Artemisinin-A Gift from traditional Chinese medicine to the world (nobel lecture). Angew Chem Int Ed Engl 55(35):10210–10226
Efferth T (2005) Mechanistic perspectives for 1,2,4-trioxanes in anti-cancer therapy. Drug Resist Updat 8(1–2):85–97
Wong YK, Xu C, Kalesh KA, He Y, Lin Q, Wong WSF, Shen HM, Wang J (2017) Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med Res Rev 37(6):1492–1517
Sun X, Yan P, Zou C, Wong YK, Shu Y, Lee YM, Zhang C, Yang ND, Wang J, Zhang J (2019) Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med Res Rev 39(6):2172–2193
Ma JD, Jing J, Wang JW, Yan T, Li QH, Mo YQ, Zheng DH, Gao JL, Nguyen KA, Dai L (2019) A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther 21(1):153
Jiang W, Cen Y, Song Y, Li P, Qin R, Liu C, Zhao Y, Zheng J, Zhou H (2016) Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine 23(11):1259–1266
Chen Y, Yan Y, Liu H, Qiu F, Liang CL, Zhang Q, Huang RY, Han L, Lu C, Dai Z (2020) Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8(+) T-cell memory in wild-type and humanized mice. Theranostics 10(23):10466–10482
Zhang F, Ma Q, Xu Z, Liang H, Li H, Ye Y, Xiang S, Zhang Y, Jiang L, Hu Y et al (2017) Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer Res 36(1):68
Dolivo D, Weathers P, Dominko T (2021) Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics. Acta Pharm Sin B 11(2):322–339
He Y, Sun B, Chen G, Huang R (2019) Dihydroartemisinin ameliorates balloon injury-induced neointimal formation in rats. J Cell Physiol 234(7):11545–11554
Wang X, Wu J, Zhang H, Sun B, Huang R (2021) Dihydroartemisinin ameliorates balloon injury-induced neointimal formation through suppressing autophagy in vascular smooth muscle cells. Biol Chem 402(4):451–460
Wang HY, Huang RP, Han P, Xue DB, Li HB, Liu B, Shan P, Wang QS, Li KS, Li HL (2014) The effects of artemisinin on the proliferation and apoptosis of vascular smooth muscle cells of rats. Cell Biochem Funct 32(2):201–208
Petrasheskaya N, Tae HJ, Ahmet I, Talan MI, Lakatta EG, Lin L (2016) A rat carotid balloon injury model to test anti-vascular remodeling therapeutics. J Vis Exp 115:53777
Zhang W, Trebak M (2014) Vascular balloon injury and intraluminal administration in rat carotid artery. J Vis Exp 94:e52045
Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW (2006) Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med 16(1):25–28
Zhang J, McIntosh BE, Wang B, Brown ME, Probasco MD, Webster S, Duffin B, Zhou Y, Guo LW, Burlingham WJ et al (2019) A human pluripotent stem cell-based screen for smooth muscle cell differentiation and maturation identifies inhibitors of intimal hyperplasia. Stem Cell Rep 12(6):1269–1281
Lim S, Kim DG, Kim S (2019) ERK-dependent phosphorylation of the linker and substrate-binding domain of HSP70 increases folding activity and cell proliferation. Exp Mol Med 51(9):1–14
Albakova Z, Armeev GA, Kanevskiy LM, Kovalenko EI, Sapozhnikov AM (2020) HSP70 multi-functionality in cancer. Cells 9(3):587
Lin Y, Wu T, Yang M, Duangmano S, Chaiwongsa R, Pornprasert S, He T (2020) Upregulation of long noncoding RNA FERRE promoted growth and invasion of breast cancer through modulating miR-19a-5p/EZH2 axis. Eur Rev Med Pharmacol Sci 24(21):11154–11164
Yang CB, Xiao SW, Cheng SM, Zhang C (2020) LncRNA FAS-AS1 inhibits the progression of non-small cell lung cancer through regulating miR-19a-5p. Eur Rev Med Pharmacol Sci 24(7):3775–3785
Kochan-Jamrozy K, Kroliczewski J, Moszynska A, Collawn JF, Bartoszewski R (2019) miRNA networks modulate human endothelial cell adaptation to cyclic hypoxia. Cell Signal 54:150–160
Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C et al (2015) Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 10(2):170–177
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L (2014) Complementary sequence-mediated exon circularization. Cell 159(1):134–147
Yang Z, Xie L, Han L, Qu X, Yang Y, Zhang Y, He Z, Wang Y, Li J (2017) Circular RNAs: regulators of cancer-related signaling pathways and potential diagnostic biomarkers for human cancers. Theranostics 7(12):3106–3117
Chen LL, Yang L (2015) Regulation of circRNA biogenesis. RNA Biol 12(4):381–388
Wang Y, Mo Y, Gong Z, Yang X, Yang M, Zhang S, Xiong F, Xiang B, Zhou M, Liao Q et al (2017) Circular RNAs in human cancer. Mol Cancer 16(1):25
Xu X, Zhang J, Tian Y, Gao Y, Dong X, Chen W, Yuan X, Yin W, Xu J, Chen K et al (2020) CircRNA inhibits DNA damage repair by interacting with host gene. Mol Cancer 19(1):128
Huang G, Liang M, Liu H, Huang J, Li P, Wang C, Zhang Y, Lin Y, Jiang X (2020) CircRNA hsa_circRNA_104348 promotes hepatocellular carcinoma progression through modulating miR-187-3p/RTKN2 axis and activating Wnt/beta-catenin pathway. Cell Death Dis 11(12):1065
Liu Y, Yang Y, Wang Z, Fu X, Chu XM, Li Y, Wang Q, He X, Li M, Wang K et al (2020) Insights into the regulatory role of circRNA in angiogenesis and clinical implications. Atherosclerosis 298:14–26
He X, Deng J, Yu XJ, Yang S, Yang Y, Zang WJ (2020) Activation of M3AChR (Type 3 Muscarinic Acetylcholine Receptor) and Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) signaling by choline alleviates vascular smooth muscle cell phenotypic switching and vascular remodeling. Arterioscler Thromb Vasc Biol 40(11):2649–2664
Huang C, Zhao J, Zhu Y (2020) Drug-eluting stent targeting SP-1-attenuated restenosis by engaging YAP-mediated vascular smooth muscle cell phenotypic modulation. J Am Heart Assoc 9(1):e014103
Li X, Ding J, Wang X, Cheng Z, Zhu Q (2020) NUDT21 regulates circRNA cyclization and ceRNA crosstalk in hepatocellular carcinoma. Oncogene 39(4):891–904
Ren S, Xu Y: AC016405.3, a novel long noncoding RNA, acts as a tumor suppressor through modulation of TET2 by microRNA-19a-5p sponging in glioblastoma. Cancer Sci 2019, 110(5):1621–1632.
Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA (2013) Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128(18):2047–2057
Funding
This work was supported by the Heilongjiang Provincial Natural Science Foundation of China (LH2020H035); the Fundamental Research Funds for the Provincial University (2019-KYYWF-0343); the open Fund of Key Laboratory of Hepatosplenic Surgery, Ministry of Education, Harbin, China (GPKF202001); Heilongjiang Postdoctoral Scientific Research Developmental Fund (LBH-Q20143); and Special supported scientific research project of the Fourth Affiliated Hospital of Harbin Medical University (HYDSYTB202101).
Author information
Authors and Affiliations
Contributions
HR designed, analyzed and interpreted the experiments, performed the VBI model in vivo and wrote the manuscript. XW performed cell culture, cell behavior experiments and the VBI model in vivo assays. WX led the study, supervised the overall project and reviewed the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, R., Xing, W. & Wang, X. Dihydroartemisinin inhibits restenosis after balloon angioplasty via circHSPA4/miR-19a-5p axis. Mol Cell Biochem 479, 951–961 (2024). https://doi.org/10.1007/s11010-023-04778-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04778-0