Abstract
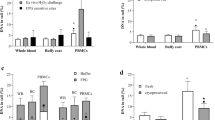
Global estimates exhibit that one million people have end-stage renal disease, a disease-state characterized by irreversible loss of kidney structure and function, thus necessitating renal replacement therapy. The disease-state, oxidative stress, inflammatory responses, as well as the treatment procedure can have damaging effects on the genetic material. Therefore, the present study was carried out to investigate DNA damage (basal and oxidative) using the comet assay in peripheral blood leukocytes of patients (n = 200) with stage V Chronic Kidney Disease (on dialysis and those recommended but yet to initiate dialysis) and compare it to that in controls (n = 210). Basal DNA damage was significantly elevated (1.13x, p ≤ 0.001) in patients (46.23 ± 0.58% DNA in tail) compared to controls (40.85 ± 0.61% DNA in tail). Oxidative DNA damage was also significantly (p ≤ 0.001) higher in patients (9.18 ± 0.49 vs. 2.59 ± 0.19% tail DNA) compared to controls. Twice-a-week dialysis regimen patients had significantly elevated % tail DNA and Damage Index compared to the non-dialyzed and to the once-a-week dialysis group implying dialysis- induced mechanical stress and blood–dialyzer membrane interactions as probable contributors to elevated DNA damage. The present study with a statistically significant power implies higher disease-associated as well as maintenance therapy (hemodialysis)-induced basal and oxidatively damaged DNA, which if not repaired has the potential to initiate carcinogenesis. These findings mark the need for improvement and development of interventional therapies for delaying disease progression and associated co-morbidities so as to improve life expectancy of patients with kidney disease.
Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, Saleem MA, Walz G, Benzing T (2003) Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet 12:3397–3405
Fogo AB (2007) Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 22:2011–2022
Cachofeiro V, Goicochea M, deVinuesa SG, Oubiña P, Lahera V, Luño J (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int 111:S4–S9
Sundaram SPM, Nagarajan S, Devi AJM (2014) Chronic kidney disease-effect of oxidative stress. Chin J Biol. https://doi.org/10.1155/2014/216210
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW (2003) Kidney disease as a risk factor for development of cardiovascular disease a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108:2154–2169
Nusair MB, Rajpurohit N, Alpert MA (2012) Chronic inflammation and coronary atherosclerosis in patients with end-stage renal disease. Cardiorenal Med 2:117–124
Baigent C, Burbury K, Wheeler D (2000) Premature cardiovascular disease in chronic renal failure. Lancet 356:147–152
Goicoechea M, deVinuesa SG, Gomez-Campdera F, Luno J (2005) Predictive cardiovascular risk factors in patients with chronic kidney disease (CKD). Kidney Int. Suppl. 93:S35–S38
Schiffrin EL, Lipman ML, Mann JF (2007) Chronic kidney disease: effects on the cardiovascular system. Circulation 116:85–97
Tsirpanlis G, Bagos P, Ioannou D (2004) Exploring inflammation in hemodialysis patients: persistent and superimposed inflammation. A longitudinal study. Kidney Blood Press Res 27:63–70
Teschner M, Garte C, Rückle-Lanz H, Mäder U, Stopper H, Klassen A, Heidland A (2002) Incidence and spectrum of malignant disease among dialysis patients in North Bavaria. Dtsch Med Wochenschr 127:2497–2502
Vajdic CM, McDonald SP, McCredie MR, VanLeeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE (2006) Cancer incidence before and after kidney transplantation. J Am Med Assoc 296:2823–2831
Smith A. 2011. Causes of renal disease. Accessed on March 18, 2016.
Roberts MA, Hare DL, Ratnaike S, Ierino FL (2006) Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis 48:341–360
Rangel-López A, Paniagua-Medina ME, Urbán-Reyes M, Cortes-Arredondo M, Alvarez-Aguilar C, López-Meza J, Ochoa-Zarzosa A, Lindholm B, García-López E, Paniagua JR (2013) Genetic damage in patients with chronic kidney disease, peritoneal dialysis and haemodialysis: a comparative study. Mutagenesis 28:219–225
Schupp N, Stopper H, Rutkowski P, Kobras K, Nebel M, Bahner U, Vienken J, Heidland A (2006) Effect of different hemodialysis regimens on genomic damage in end-stage renal failure. Semin Nephrol 26:28–32
Schupp N, Stopper H, Heidland A (2016) DNA damage in chronic kidney disease: evaluation of clinical biomarkers. Oxid Med Cell Longev 2016:3592042
Hussain SP, Harris CC (2007) Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 121:2373–2380
Abbas T, Keaton MA, Dutta A (2015) Genomic instability in cancer. Cold Spring Harbor Persp Biol 5:1–19
Schumacher B, Garinis GA, Hoeijmakers JHJ (2008) Age to survive: DNA damage and aging. Trends Genet 24:77–85
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr NeuroPharmacol 7:65–74
GBD (The Global Burden of Diseases, Injuries, and Risk Factors Study) Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395:709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Cooke MS, Loft S, Olinski R, Evans MD, Bialkowski K, Wagner JR, Dedon PC, Møller P, Greenberg MM, Cadet J (2010) Recommendations for standardized description of and nomenclature concerning oxidatively damaged nucleobases in DNA. Chem Res Toxicol 23:705–707. https://doi.org/10.1021/tx1000706
Chao MR, Evans MD, Hu CW, Ji Y, Møller P, Rossner P, Cooke MS (2021) Biomarkers of nucleic acid oxidation—a summary state-of-the-art. Redox Biol 42:101872. https://doi.org/10.1016/j.redox.2021.101872
Collins AR (2009) Investigating oxidative DNA damage and its repair using the comet assay. Mutat Res 681:24–32
Møller P, Stopper H, Collins AR (2020) Measurement of DNA damage with the comet assay in high-prevalence diseases: current status and future directions. Mutagenesis 35:5–18. https://doi.org/10.1093/mutage/gez018
Gandhi G, Mehta T, Contractor P, Tung G (2018) Prognostic markers of genomic instability in end stage renal disease. Mutat Res, Genet Toxicol Environ Mutagen 835:1–10
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker D, Tice R, Waters MD, Aitio A (2000) IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res 463:11–72
Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, Joshi SR, Sadikot S, Gupta R, Gulati S, Munjal YP (2009) Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 57:163–169
IGH. Definition and Classification (2013). Special issue on Indian guidelines on hypertension. JAPI (I.G.H.)-III 2013; 61: 12.
Møller P, Azqueta A, Boutet-Robinet E, Koppen G, Bonassi S, Milić M, Gajski G, Costa S, Teixeira JP, CostaPereira C, Dusinska M, Godschalk R, Brunborg G, Gutzkow KB, Giovannelli L, Cooke MS, Richling E, Laffon B, Valdiglesias V, Basaran N, Langie S (2020) Minimum information for reporting on the comet assay (MIRCA): recommendations for describing comet assay procedures and results. Nat Protoc 15:3817–3826. https://doi.org/10.1038/s41596-020-0398-1
Ahuja YR, Saran R (1999) Alkaline single cell gel electrophoresis assay. Protoc J Cytol Genet 34:57–62
Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR (2003) Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis 18:45–51
Collins A, Ai-guo M, Duthie SJ (1995) The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat Res 336:69–77
Garcia O, Romero I, González JE, Mandina T (2007) Measurements of DNA damage on silver stained comets using free Internet software. Mutat Res 5:186–190
National Kidney Foundation (2015) KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66(5):884–930
Collins A, Dušinská M, Gedik C, Štětina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104:465–469. https://doi.org/10.2307/3432805
Floyd RA (1997) Protective action of nitrone-based free radical traps against oxidative damage to the central nervous system. Adv Pharmacol 38:361–378
Kasai H (1997) Analysis of a form of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387:147–163
Freidovich I (1999) Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci 893:13–18
Ames BN (1983) Dietary carcinogens and anticarcinogens. Oxygen Radic Degener Dis Sci 221:1256–1264
Pryor WA (1987) The free-radical theory of aging revisited: A critique and a suggested disease-specific theory. In: Warner HR, Butler RN, Sprott RL, Schneider EL (eds) Modern biological theories of aging. Raven Press, New York, pp 89–112
Dusinska M, Collins AR (2008) The comet assay in human biomonitoring: gene-environment interactions. Mutagenesis 23:191–205
Lee E, Oh E, Lee J, Sul D, Lee J (2004) Use of the tail moment of the lymphocytes to evaluate DNA damage in human biomonitoring studies. Toxicol Sci 81:121–132
Kumaravel TS, Jha AN (2006) Reliable comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res 605:7–16
Cotelle S, Férard JF (1999) Comet assay in genetic ecotoxicology: a review. Environ Mol Mutagen 34:246–255
Collins AR, Oscoz AA, Brunborg G, Giovannelli LG, Kruszewski M, Smith CC, Stetina R (2008) The comet assay: topical issues. Mutagenesis 23:143–151
Muruzabal D, Sanz-Serrano J, Sauvaigo S, Gützkow KB, deCerain AL, Vettorazzi A, Azqueta A (2020) Novel approach for the detection of alkylated bases using the enzyme-modified comet assay. Toxicol Lett 330:108–117
Muruzabal D, Collins A, Azqueta A (2021) The enzyme-modified comet assay: past, present and future. Food Chem Toxicol 147:111865. https://doi.org/10.1016/j.fct.2020.111865
Stopper H, Boullay F, Heidland A, Vienken J, Bahner U (2001) Comet-assay analysis identifies genomic damage in lymphocytes of uremic patients. Am J Kidney Dis 38:296–301
Kan E, Undeğer U, Bali M, Başaran N (2002) Assessment of DNA strand breakage by the alkaline COMET assay in dialysis patients and the role of vitamin E supplementation. Mutat Res 520:151–159
Stoyanova E, Sandoval SB, Zúñiga LA, El-Yamani N, Coll E, Pastor S, Reyes J, Andrés E, Ballarin J, Xamena N, Marcos R (2010) Oxidative DNA damage in chronic renal failure patients. Nephrol Dial Transpl 25:879–885
Moffitt T, Hariton F, Devlin M, Garrett PJ, Hannon-Fletcher MPA (2014) Oxidative DNA damage is elevated in renal patients undergoing haemodialysis. Open J Prev Med 4:421–428
Kobras K, Schupp N, Nehrlich K, Adelhardt M, Bahner U, Vienken J, Heidland A, Sebekova K, Stopper H (2006) Relation between different treatment modalities and genomic damage of end-stage renal failure patients. Kidney Blood Press Res 29:10–17
Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, Kovesdy CP, Kalantar-Zadeh K (2014) Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56:415–425
Flegal KM, Graubard BI, Williamson DF, Gail MH (2007) Cause-specific excess deaths associated with underweight, overweight, and obesity. J Am Med Assoc 298:2028–2037
Huang J, Lin HY, Lim L, Chen S, Chang J, Hwang S, Tsai J, Hung C, Chen H (2015) Body mass index, mortality, and gender difference in advanced chronic kidney disease. PLoS ONE 10:e0126668
Navaneethan SD, Schold JD, Arrigain S, Kirwan JP, Nally JV (2016) Body mass index and causes of death in chronic kidney disease. Kidney Int 89:675–682
Mohsen A, Brown R, Hoefield R, Kalra PA, O’Donoghue D, Middleton R, New D (2012) Body mass index has no effect on rate of progression of chronic kidney disease in subjects with type 2 diabetes mellitus. J Nephrol 25:384–393
Kristic S, Zubovic SV, Zukic F (2013) The relationship of chronic renal failure and body mass index in patients without diabetes. Med Arch 67:406–406
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Stopper H, Meysen T, Böckenförde A, Bahner U, Heidland A, Vamvakas S (1999) Increased genomic damage in lymphocytes of patients before and after long-term maintenance hemodialysis therapy. Am J Kidney Dis 34:433–437
Gandhi G, Tung G (2017) Sensitivity and specificity prediction of the buccal micronucleus cytome assay in end-stage renal disease patients on dialysis: a case-control study. Mutat Res 822:1–9
Malachi T, Zevin D, Gafter U, Chagnac A, Slor H, Levi J (1993) DNA repair and recovery of RNA synthesis in uremic patients. Kidney Int 44:385–389
Boxall MC, Goodship TH, Brown AL, Ward MC, vonZglinicki T (2006) Telomere shortening and haemodialysis. Blood Purif 24:185–189
Oberoi SS (2015) Updating income ranges for Kuppuswamy’s socio-economic status scale for the year 2014. Indian J Public Health 59:156–157
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ (2003) Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42:1206–1252
Acknowledgements
This work has been supported by UGC research project grant sanctioned to GG and GKT is thankful for the UGC-project fellowship and the UPE fellowship from GNDU. The authors are thankful to the doctors and study participants for their cooperation.
Funding
Research funding under University Grants Commission, New Delhi, India sanctioned to Gursatej Gandhi is duly acknowledged.
Author information
Authors and Affiliations
Contributions
GG contributed to the study conception and design. Material preparation, data collection and analysis were performed by GKT. The first draft of the manuscript was written by GKT and both the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tung, G.K., Gandhi, G. Baseline and oxidatively damaged DNA in end-stage renal disease patients on varied hemodialysis regimens: a comet assay assessment. Mol Cell Biochem 479, 199–211 (2024). https://doi.org/10.1007/s11010-023-04720-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04720-4