Abstract
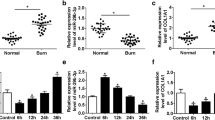
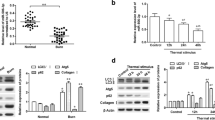
Burn injury is a serious traumatic injury that leads to severe physical and psychosocial impairment. Wound healing after burn injury is a substantial challenge in medical community. This study investigated the biological effects of the demethylase fat mass and obesity-associated protein (FTO) on burn injury. FTO protein level in burn skin tissues of patients was measured with Western blot assay. Keratinocytes (HaCaT cells) were given heat stimulation to induce an in vitro burn injury model, and then transfected with overexpression plasmids of FTO (pcDNA-FTO) or small interfering RNA against FTO (si-FTO). Cell proliferation, migration, and angiogenesis in keratinocytes were evaluated with CCK-8, Transwell, and tube formation assays, respectively. Tissue factor pathway inhibitor-2 (TFPI-2) m6A methylation level was detected with MeRIP‑qPCR assay. Then rescue experiments were conducted to explore the effects of FTO/TFPI-2 axis on keratinocyte functions. Lentivirus carrying FTO overexpression plasmids was injected into a burn rat model to detect its effects on wound healing and depressive-like behaviors in burn rats. FTO was downregulated in burn skin and heat-stimulated keratinocytes. FTO prominently augmented proliferation, migration and angiogenesis in heat-stimulated keratinocytes, while FTO knockdown showed the opposite results. FTO inhibited TFPI-2 expression by FTO-mediated m6A methylation modification. TFPI-2 overexpression abrogated FTO mediated enhancement of proliferation, migration and angiogenesis in keratinocytes. Additionally, FTO overexpression accelerated wound healing and improved depressive-like behaviors in burn rat model. FTO prominently augmented proliferation, migration and angiogenesis in heat-stimulated keratinocytes though inhibiting TFPI-2, and then improved wound healing and depressive-like behaviors.







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
Abbreviations
- FTO:
-
Fat mass and obesity-associated protein
- TFPI-2:
-
Tissue factor pathway inhibitor-2
- m6A:
-
N6‑methyladenosine
- VEGF:
-
Vascular endothelial growth factor
References
Wang Y, Beekman J, Hew J et al (2018) Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev 123:3–17. https://doi.org/10.1016/j.addr.2017.09.018
Markiewicz-Gospodarek A, Kozioł M, Tobiasz M et al (2022) Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph19031338
Nelson S, Conroy C, Logan D (2019) The biopsychosocial model of pain in the context of pediatric burn injuries. Eur J Pain 23:421–434. https://doi.org/10.1002/ejp.1319
Shi H, Cheer K, Simanainen U et al (2021) The contradictory role of androgens in cutaneous and major burn wound healing. Burns Trauma 9:0tkaa46. https://doi.org/10.1093/burnst/tkaa046
Du H, Zhou Y, Suo Y et al (2018) CCN1 accelerates re-epithelialization by promoting keratinocyte migration and proliferation during cutaneous wound healing. Biochem Biophys Res Commun 505:966–972. https://doi.org/10.1016/j.bbrc.2018.09.001
Azzam SK, Alsafar H, Sajini AA (2022) FTO m6A demethylase in obesity and cancer: implications and underlying molecular mechanisms. Int J Mol Sci. https://doi.org/10.3390/ijms23073800
Kang H, Zhang Z, Yu L et al (2018) FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J Cell Biochem 119:5676–5685. https://doi.org/10.1002/jcb.26746
Xu Y, Ye S, Zhang N et al (2020) The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun (Lond) 40:484–500. https://doi.org/10.1002/cac2.12075
Zhang L, Wan Y, Zhang Z et al (2021) FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway. RNA Biol 18:1265–1278. https://doi.org/10.1080/15476286.2020.1841458
Cui YH, Yang S, Wei J et al (2021) Autophagy of the m(6)A mRNA demethylase FTO is impaired by low-level arsenic exposure to promote tumorigenesis. Nat Commun 12:2183. https://doi.org/10.1038/s41467-021-22469-6
Chang R, Huang Z, Zhao S et al (2022) Emerging roles of FTO in neuropsychiatric disorders. Biomed Res Int 2022:2677312. https://doi.org/10.1155/2022/2677312
Zarza-Rebollo JA, Molina E, Rivera M (2021) The role of the FTO gene in the relationship between depression and obesity. A systematic review. Neurosci Biobehav Rev 127:630–637. https://doi.org/10.1016/j.neubiorev.2021.05.013
Wang XL, Wei X, Yuan JJ et al (2022) Downregulation of fat mass and obesity-related protein in the anterior cingulate cortex participates in anxiety- and depression-like behaviors induced by neuropathic pain. Front Cell Neurosci 16:884296. https://doi.org/10.3389/fncel.2022.884296
Lavergne M, Guillon-Munos A, Lenga Ma Bonda W et al (2021) Tissue factor pathway inhibitor 2 is a potent kallikrein-related protease 12 inhibitor. Biol Chem 402:1257–1268. https://doi.org/10.1515/hsz-2020-0389
Liu J, Xie J, Huang Y et al (2021) TFPI-2 inhibits the invasion and metastasis of bladder cancer cells. Prog Urol 31:71–77. https://doi.org/10.1016/j.purol.2020.07.243
Wang G, Huang W, Li W et al (2018) TFPI-2 suppresses breast cancer cell proliferation and invasion through regulation of ERK signaling and interaction with actinin-4 and myosin-9. Sci Rep 8:14402. https://doi.org/10.1038/s41598-018-32698-3
Zheng L, Huang J, Su Y et al (2020) Overexpression of tissue factor pathway inhibitor 2 attenuates trophoblast proliferation and invasion in preeclampsia. Hum Cell 33:512–520. https://doi.org/10.1007/s13577-020-00322-0
Xu Z, Maiti D, Kisiel W et al (2006) Tissue factor pathway inhibitor-2 is upregulated by vascular endothelial growth factor and suppresses growth factor-induced proliferation of endothelial cells. Arterioscler Thromb Vasc Biol 26:2819–25. https://doi.org/10.1161/01.atv.0000248731.55781.87
Zhi S, Li J, Kong X et al (2021) Insulin-like growth factor 2 mRNA binding protein 2 regulates proliferation, migration, and angiogenesis of keratinocytes by modulating heparanase stability. Bioengineered 12:11267–11276. https://doi.org/10.1080/21655979.2021.2002495
Zhou X, Ning K, Ling B et al (2019) Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cells Dev 28:1463–1472. https://doi.org/10.1089/scd.2019.0113
Li X, Wang H, Chen Q et al (2019) Felbamate produces antidepressant-like actions in the chronic unpredictable mild stress and chronic social defeat stress models of depression. Fundam Clin Pharmacol 33:621–633. https://doi.org/10.1111/fcp.12466
Wu Z, You Z, Chen P et al (2018) Matrine exerts antidepressant-like effects on mice: role of the hippocampal PI3K/Akt/mTOR signaling. Int J Neuropsychopharmacol 21:764–776. https://doi.org/10.1093/ijnp/pyy028
Khan A, Shal B, Naveed M et al (2020) Matrine alleviates neurobehavioral alterations via modulation of JNK-mediated caspase-3 and BDNF/VEGF signaling in a mouse model of burn injury. Psychopharmacology (Berl) 237:2327–2343. https://doi.org/10.1007/s00213-020-05537-5
Wang Z, Chen L, Rong X et al (2017) Upregulation of MAOA in the hippocampus results in delayed depressive-like behaviors in burn mice. Burns. https://doi.org/10.1016/j.burns.2017.03.013
Rennekampff HO, Alharbi Z (2021) Burn injury: mechanisms of keratinocyte cell death. Med Sci (Basel). https://doi.org/10.3390/medsci9030051
Hosseini Mansoub N (2021) The role of keratinocyte function on the defected diabetic wound healing. Int J Burns Trauma 11:430–441
Sun L, Ma L, Zhang H et al (2019) Fto deficiency reduces anxiety-and depression-like behaviors in mice via alterations in gut microbiota. Theranostics 9:721–733. https://doi.org/10.7150/thno.31562
Wang W, He Y, Zhai LL et al (2022) m(6)A RNA demethylase FTO promotes the growth, migration and invasion of pancreatic cancer cells through inhibiting TFPI-2. Epigenetics 1–15. https://https://doi.org/10.1080/15592294.2022.2061117
Ghilardi C, Anastasia A, Avigni R et al (2016) PO-44 - Tissue factor pathway inhibitor-2 (TFPI-2) is cleaved by PRSS3: implication for tumor endothelial cells migration. Thromb Res 140(Suppl 1):S192-3. https://doi.org/10.1016/s0049-3848(16)30177-3
Ivanciu L, Gerard RD, Tang H et al (2007) Adenovirus-mediated expression of tissue factor pathway inhibitor-2 inhibits endothelial cell migration and angiogenesis. Arterioscler Thromb Vasc Biol 27:310–6. https://doi.org/10.1161/01.ATV.0000254147.89321.cf
Acknowledgements
We acknowledge the supports of fundings: Xi'an Science and Technology Plan Project, 2019115013YX005SF038 (6) and the Natural Science Basic Research Program of Shaanxi, 2022JQ-975.
Funding
This research is supported by Xi'an Science and Technology Plan Project, 2019115013YX005SF038 (6) and the Natural Science Basic Research Program of Shaanxi, 2022JQ-975.
Author information
Authors and Affiliations
Contributions
Xu ZH: Methodology, Investigation, Data curation, Original draft. Zhu XM: Methodology, Investigation, Data curation, Original draft. Mu SZ: Review & editing. Fan RH: Writing, Review & editing. Wang BF: Investigation. Gao WJ: Writing. Kang T: Idea, Supervision, Review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study has approved by the Ethics Committees of the Shaanxi Provincial People’s Hospital and abided by the ethical guidelines of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Z., Zhu, X., Mu, S. et al. FTO overexpression expedites wound healing and alleviates depression in burn rats through facilitating keratinocyte migration and angiogenesis via mediating TFPI-2 demethylation. Mol Cell Biochem 479, 325–335 (2024). https://doi.org/10.1007/s11010-023-04719-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04719-x