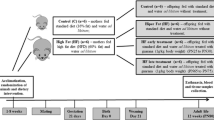
Abstract
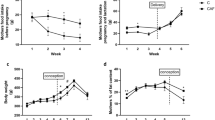
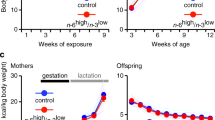
CD36 and GPR120 play an important role in the perception and preference for fat-rich food consumption. We aimed to investigate the relationship between oro-gustatory perception of lipids, fatty taste preference, and maternal (Gestation + Lactation)-maturation period nutrition status in offspring Sprague–Dawley rats. In our study, mother rats were fed with control (C) or high-fat diets (HFD) during gestation (21 days) and lactation (21 days) periods. After weaning, the offspring were fed with control (C) or high-fat diets (HFD) during the maturation (120 days) period. Daily calorie intake and weekly body weight measurements were monitored. Two-bottle preference (TBPT) and licking tests measured the fat perceptions and preferences. Plasma levels of insulin, leptin, glucose, and triglyceride were measured. The protein and mRNA expressions of CD36 and GPR120 in the circumvallate papillae (CVP) were determined. The 48 h TBPT results revealed that maternal HFD-exposed offspring rats significantly preferred 2% rapeseed oil solution regardless of the type of maturation diet. According to the licking test, C/C group (C diet exposed group in maternal and maturation periods) offspring licked 0.1% oleic acid-containing water more than C/HFD (C diet exposed in maternal period and HFD exposed group in maturation period) and HFD/HFD group. (HFD exposed group in maternal and maturation periods) groups. Plasma insulin and leptin concentrations significantly increased in HFD/HFD groups compared to C/C group. CD36 protein expressions were significantly lower in HFD/HFD than C/HFD and HFD/C groups. GPR120 and GNAT3 mRNA expressions in HFD/C group were significantly higher than in C/HFD group. Our results suggest that HFD exposure during maternal and maturation period may play a role in fat perception/preference through oral lipid sensors.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Passilly-Degrace P, Gaillard D, Besnard P (2009) Orosensory perception of dietary lipids in mammals. Results Probl Cell Differ 47:221–238. https://doi.org/10.1007/400_2008_7
Drewnowski A (1997) Taste preferences and food intake. Annu Rev Nutr 17:237–253. https://doi.org/10.1146/annurev.nutr.17.1.237
Semerciöz AS, Yılmaz B, Özilgen M (2020) Thermodynamic assessment of allocation of energy and exergy of the nutrients for the life processes during pregnancy. Br J Nutr 124:742–753. https://doi.org/10.1017/s0007114520001646
Khan NA, Besnard P (2009) Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta 1791:149–155. https://doi.org/10.1016/j.bbalip.2009.01.001
Kulkarni BV, Mattes RD (2014) Lingual lipase activity in the orosensory detection of fat by humans. Am J Physiol Regul Integr Comp Physiol 306:R879–R885. https://doi.org/10.1152/ajpregu.00352.2013
Reed DR, Xia MB (2015) Recent advances in fatty acid perception and genetics. Adv Nutr 6:353S-S360. https://doi.org/10.3945/an.114.007005
Miyamoto J, Hasegawa S, Kasubuchi M, Ichimura A, Nakajima A, Kimura I (2016) Nutritional signaling via free fatty acid receptors. Int J Mol Sci 17:450. https://doi.org/10.3390/ijms17040450
Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P (2005) CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115:3177–3184. https://doi.org/10.1172/JCI25299
Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA (2014) CD36- and GPR120-mediated Ca(2)(+) signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 146:995–1005. https://doi.org/10.1053/j.gastro.2014.01.006
Chen CS, Bench EM, Allerton TD, Schreiber AL, Arceneaux KP 3rd, Primeaux SD (2013) Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regul Integr Comp Physiol 305:R1346–R1355. https://doi.org/10.1152/ajpregu.00582.2012
Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, Liu XM (2011) Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem 113:663–667. https://doi.org/10.1016/j.acthis.2010.09.007
Ventura AK, Worobey J (2013) Early influences on the development of food preferences. Curr Biol 23:R401–R408. https://doi.org/10.1016/j.cub.2013.02.037
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM (2010) Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151:4756–4764. https://doi.org/10.1210/en.2010-0505
Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF (2008) Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28:12107–12119. https://doi.org/10.1523/JNEUROSCI.2642-08.2008
Ong ZY, Muhlhausler BS (2011) Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J 25:2167–2179. https://doi.org/10.1096/fj.10-178392
Gugusheff JR, Bae SE, Rao A, Clarke IJ, Poston L, Taylor PD, Coen CW, Muhlhausler BS (2016) Sex and age-dependent effects of a maternal junk food diet on the mu-opioid receptor in rat offspring. Behav Brain Res 301:124–131. https://doi.org/10.1016/j.bbr.2015.12.027
Kutlu S, Aydin M, Alcin E, Ozcan M, Bakos J, Jezova D, Yilmaz B (2010) Leptin modulates noradrenaline release in the paraventricular nucleus and plasma oxytocin levels in female rats: a microdialysis study. Brain Res 1317:87–91. https://doi.org/10.1016/j.brainres.2009.12.044
Aklan I, Sayar Atasoy N, Yavuz Y, Ates T, Coban I, Koksalar F, Filiz G, Topcu IC, Oncul M, Dilsiz P, Cebecioglu U, Alp MI, Yilmaz B, Davis DR, Hajdukiewicz K, Saito K, Konopka W, Cui H, Atasoy D (2020) NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab 31:313-326 e5. https://doi.org/10.1016/j.cmet.2019.11.016
Yilmaz B, Gilmore DP, Wilson CA (1996) Inhibition of the pre-ovulatory LH surge in the rat by central noradrenergic mediation: involvement of an anaesthetic (urethane) and opioid receptor agonists. Biog Amines 12:423–435
Kutlu S, Yilmaz B, Canpolat S, Sandal S, Ozcan M, Kumru S, Kelestimur H (2004) Mu opioid modulation of oxytocin secretion in late pregnant and parturient rats. Involve Noradrenergic Neurotrans Neuroendocrinol 79:197–203. https://doi.org/10.1159/000078101
Peterschmitt Y, Abdoul-Azize S, Murtaza B, Barbier M, Khan AS, Millot JL, Khan NA (2018) Fatty acid lingual application activates gustatory and reward brain circuits in the mouse. Nutrients. https://doi.org/10.3390/nu10091246
Canpolat S, Tug N, Seyran AD, Kumru S, Yilmaz B (2010) Effects of raloxifene and estradiol on bone turnover parameters in intact and ovariectomized rats. J Physiol Biochem 66:23–28. https://doi.org/10.1007/s13105-010-0008-8
Barlow LA (2015) Progress and renewal in gustation: new insights into taste bud development. Development 142:3620–3629. https://doi.org/10.1242/dev.120394
Besnard P, Passilly-Degrace P, Khan NA (2016) Taste of fat: a sixth taste modality? Physiol Rev 96:151–176. https://doi.org/10.1152/physrev.00002.2015
Gaillard D, Stratford JM (2016) Measurement of behavioral taste responses in mice: two-bottle preference, lickometer, and conditioned taste-aversion tests. Curr Protoc Mouse Biol 6:380–407. https://doi.org/10.1002/cpmo.18
Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S (2010) Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30:8376–8382. https://doi.org/10.1523/JNEUROSCI.0496-10.2010
Chevrot M, Bernard A, Ancel D, Buttet M, Martin C, Abdoul-Azize S, Merlin JF, Poirier H, Niot I, Khan NA, Passilly-Degrace P, Besnard P (2013) Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J Lipid Res 54:2485–2494. https://doi.org/10.1194/jlr.M039446
Hayar A, Bryant JL, Boughter JD, Heck DH (2006) A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. J Neurosci Methods 153:203–207. https://doi.org/10.1016/j.jneumeth.2005.10.023
Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P (2011) The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE 6:e24014. https://doi.org/10.1371/journal.pone.0024014
McCormack DN, Clyburn VL, Pittman DW (2006) Detection of free fatty acids following a conditioned taste aversion in rats. Physiol Behav 87:582–594. https://doi.org/10.1016/j.physbeh.2005.12.004
Birsen I, Gemici B, Acar N, Ustunel I, Izgut-Uysal VN (2017) The role of apelin in the healing of water-immersion and restraint stress-induced gastric damage. J Physiol Sci 67:373–385. https://doi.org/10.1007/s12576-016-0469-9
Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, Fushiki T (2007) GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res 28:49–55. https://doi.org/10.2220/biomedres.28.49
Li L, Zhi D, Cheng R, Li J, Luo C, Li H (2021) The neuroprotective role of SIRT1/PGC-1alpha signaling in limb postconditioning in cerebral ischemia/reperfusion injury. Neurosci Lett 749:135736. https://doi.org/10.1016/j.neulet.2021.135736
Gugusheff JR, Ong ZY, Muhlhausler BS (2015) The early origins of food preferences: targeting the critical windows of development. FASEB J 29:365–373. https://doi.org/10.1096/fj.14-255976
Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279:E83–E87. https://doi.org/10.1152/ajpendo.2000.279.1.E83
Blundell JE, MacDiarmid JI (1997) Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc 97:S63–S69. https://doi.org/10.1016/s0002-8223(97)00733-5
Gugusheff JR, Vithayathil M, Ong ZY, Muhlhausler BS (2013) The effects of prenatal exposure to a “junk food” diet on offspring food preferences and fat deposition can be mitigated by improved nutrition during lactation. J Dev Orig Health Dis 4:348–357. https://doi.org/10.1017/S2040174413000330
Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD (2011) Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 176:225–236. https://doi.org/10.1016/j.neuroscience.2010.12.037
Gaillard D, Passilly-Degrace P, Besnard P (2008) Molecular mechanisms of fat preference and overeating. Ann N Y Acad Sci 1141:163–175. https://doi.org/10.1196/annals.1441.028
Treesukosol Y, Sun B, Moghadam AA, Liang NC, Tamashiro KL, Moran TH (2014) Maternal high-fat diet during pregnancy and lactation reduces the appetitive behavioral component in female offspring tested in a brief-access taste procedure. Am J Physiol Regul Integr Comp Physiol 306:R499-509. https://doi.org/10.1152/ajpregu.00419.2013
Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL (2012) Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 61:2833–2841. https://doi.org/10.2337/db11-0957
Howie GJ, Sloboda DM, Kamal T, Vickers MH (2009) Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 587:905–915. https://doi.org/10.1113/jphysiol.2008.163477
Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH (2009) Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58:1116–1125. https://doi.org/10.2337/db08-1129
Wang L, Xu F, Zhang XJ, Jin RM, Li X (2015) Effect of high-fat diet on cholesterol metabolism in rats and its association with Na(+)/K(+)-ATPase/Src/pERK signaling pathway. J Huazhong Univ Sci Technolog Med Sci 35:490–494. https://doi.org/10.1007/s11596-015-1458-6
Ehehalt R, Fullekrug J, Pohl J, Ring A, Herrmann T, Stremmel W (2006) Translocation of long chain fatty acids across the plasma membrane–lipid rafts and fatty acid transport proteins. Mol Cell Biochem 284:135–140. https://doi.org/10.1007/s11010-005-9034-1
Tekin S, Erden Y, Sandal S, Etem Onalan E, Ozyalin F, Ozen H, Yilmaz B (2017) Effects of apelin on reproductive functions: relationship with feeding behavior and energy metabolism. Arch Physiol Biochem 123:9–15. https://doi.org/10.1080/13813455.2016.1211709
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. https://doi.org/10.1038/nrg3185
Wong GT, Gannon KS, Margolskee RF (1996) Transduction of bitter and sweet taste by gustducin. Nature 381:796–800. https://doi.org/10.1038/381796a0
Kisioglu B, Nergiz-Unal R (2020) Potential effect of maternal dietary sucrose or fructose syrup on CD36, leptin, and ghrelin-mediated fetal programming of obesity. Nutr Neurosci 23:210–220. https://doi.org/10.1080/1028415X.2018.1491151
Song L, Chen K, Yan J, Zhang Y, Mao X, Lu B, Sun B (2019) Maternal high-fat diet during gestation and lactation increases conditioned aversion threshold for sucrose and alters sweet taste receptors expression in taste buds in rat offspring. Physiol Behav 212:112709. https://doi.org/10.1016/j.physbeh.2019.112709
Yilmaz B, Kutlu S, Canpolat S, Sandal S, Ayar A, Mogulkoc R, Kelestimur H (2001) Effects of paint thinner exposure on serum LH, FSH and testosterone levels and hypothalamic catecholamine contents in the male rat. Biol Pharm Bull 24:163–166. https://doi.org/10.1248/bpb.24.163
Dilsiz P, Aklan I, Sayar Atasoy N, Yavuz Y, Filiz G, Koksalar F, Ates T, Oncul M, Coban I, Ates Oz E, Cebecioglu U, Alp MI, Yilmaz B, Atasoy D (2020) MCH neuron activity is sufficient for reward and reinforces feeding. Neuroendocrinology 110:258–270. https://doi.org/10.1159/000501234
Khan AS, Subramaniam S, Dramane G, Khelifi D, Khan NA (2017) ERK1 and ERK2 activation modulates diet-induced obesity in mice. Biochimie 137:78–87. https://doi.org/10.1016/j.biochi.2017.03.004
Acknowledgements
We thank Dr. Deniz Atasoy (Iowa University, Department of Neuroscience and Pharmacology) for his technical advice in designing licking tests.
Funding
This project is supported by The Scientific and Technological Research Council of Turkey (TUBITAK) Project number: 117S384.
Author information
Authors and Affiliations
Contributions
EG: performed experiments, analyzed the results and assisted in writing the manuscript. MEK, CCC and AG: assisted in performing the experiments. VAB and CSE: assisted in writing the manuscript. BY: supervised the project and the manuscript. BG: designed the project, found the budget from TUBITAK, supervised the experimental procedure and experiments, analyzed the data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Ethical approval
The study protocol was approved by the Yeditepe University Experimental Animal Ethics Committee (protocol # 17.12.2016.575).
Consent to participate
Not Applicable.
Consent to publish
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Günalan, E., Karagöz, M.E., Cıvaş, C.C. et al. The effect of maternal period nutritional status on oro-sensorial fat perception and taste preference in rats. Mol Cell Biochem 478, 2861–2873 (2023). https://doi.org/10.1007/s11010-023-04703-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04703-5