Abstract
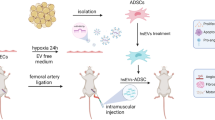
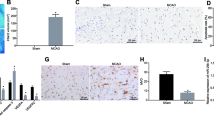
Hypoxic mesenchymal stem cell-derived extracellular vesicles (EVs) have been suggested as a promising therapy for various diseases. This study aims to determine the effect of EVs derived from bone marrow mesenchymal stem cells (BMMSCs) under hypoxia on lower limb ischemia and the underlying mechanism. Human BMMSCs were subjected to hypoxia or normoxia followed by the isolation of EVs. Nanoparticle trafficking analysis (NTA), transmission electron microscopy (TEM), and Western Blotting using corresponding markers were performed to confirm the EVs. The EVs from BMMSCs under hypoxia condition (Hyp-EVs) or normoxia condition (Nor-EVs) were subjected to hindlimb ischemia (HI) mice. MiR-34c expression in BMMSCs and BMMSC-EVs was detected. The role of miR-34c in regulating M2 macrophage polarization, as well as the target of miR-34c, were explored. HI mice with Hyp-EV treatment, as compared to the Nor-EV or the PBS group, had better blood flow and higher capillary density. MiR-34c expression was increased in BMMSCs, BMMSC-EVs, and the adductor muscle of HI mice. Hyp-EVs promoted the M2 macrophage polarization and anti-inflammatory cytokine production, and enhanced the blood flow and capillary density in HI mice, while the knockdown of miR-34c partly reversed these effects. PTEN is a target of miR-34c, and the PTEN silencing facilitated M2 macrophage polarization, whereas the inhibition of AKT signaling partly abolished the effect. Hyp-EVs promoted M2 macrophage polarization by delivering miR-34c via PTEN/AKT pathway, which could be a promising therapeutic strategy to ameliorate lower limb ischemia.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Criqui MH, Aboyans V (2015) Epidemiology of peripheral artery disease. Circ Res 116(9):1509–1526
Espinola-Klein C, Savvidis S (2009) Peripheral arterial disease: epidemiology, symptoms and diagnosis. Internist (Berl) 50(8):919–926
Van Pham P, Vu NB, Nguyen HT, Dao TT, Le HT, Phi LT, Nguyen OT, Phan NK (2017) ETV-2 activated proliferation of endothelial cells and attenuated acute hindlimb ischemia in mice. In Vitro Cell Dev Biol Anim 53(7):616–625
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229(2):176–185
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233(9):6425–6440
Tepekoylu C, Lobenwein D, Urbschat A, Graber M, Pechriggl EJ, Fritsch H, Paulus P, Grimm M, Holfeld J (2018) Shock wave treatment after hindlimb ischaemia results in increased perfusion and M2 macrophage presence. J Tissue Eng Regen Med 12(1):e486–e494
Zhu D, Johnson TK, Wang Y, Thomas M, Huynh K, Yang Q, Bond VC, Chen YE, Liu D (2020) Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther 11(1):162
Zhang J, Muri J, Fitzgerald G, Gorski T, Gianni-Barrera R, Masschelein E, D’Hulst G, Gilardoni P, Turiel G, Fan Z et al (2020) Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab 31(6):1136–1153
Hsiao LC, Carr C, Chang KC, Lin SZ, Clarke K (2013) Stem cell-based therapy for ischemic heart disease. Cell Transpl 22(4):663–675
Quiroz HJ, Valencia SF, Liu ZJ, Velazquez OC (2020) Increasing the Therapeutic Potential of Stem Cell Therapies for Critical Limb Ischemia. HSOA J Stem Cells Res Dev Ther 6:1
Chugh AR, Zuba-Surma EK, Dawn B (2009) Bone marrow-derived mesenchymal stems cells and cardiac repair. Minerva Cardioangiol 57(2):185–202
Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S (2005) Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 66(3):543–551
Gremmels H, Teraa M, Quax PH, den Ouden K, Fledderus JO, Verhaar MC (2014) Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther 22(11):1960–1970
Xiang Q, Hong D, Liao Y, Cao Y, Liu M, Pang J, Zhou J, Wang G, Yang R, Wang M et al (2017) Overexpression of gremlin1 in mesenchymal stem cells improves hindlimb ischemia in mice by enhancing cell survival. J Cell Physiol 232(5):996–1007
Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25(10):2648–2659
Jackson WM, Nesti LJ, Tuan RS (2012) Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 1(1):44–50
Zhang J, Rong Y, Luo C, Cui W (2020) Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging (Albany NY) 12(24):25138–25152
Li R, Zhao K, Ruan Q, Meng C, Yin F (2020) Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther 22(1):75
Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, Zhao X, Lu C (2019) Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res 38(1):62
Daly M, O’Driscoll L (2017) MicroRNA Profiling of Exosomes. Methods Mol Biol 1509:37–46
Kang HJ, Kang WS, Hong MH, Choe N, Kook H, Jeong HC, Kang J, Hur J, Jeong MH, Kim YS et al (2015) Involvement of miR-34c in high glucose-insulted mesenchymal stem cells leads to inefficient therapeutic effect on myocardial infarction. Cell Signal 27(11):2241–2251
Wan FZ, Chen KH, Sun YC, Chen XC, Liang RB, Chen L, Zhu XD (2020) Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma. J Transl Med 18(1):12
Wei G, Liao C, Jian C, Liang L, Liu J, Tang Y, Wei Y (2020) Evaluation of miR-34b/c polymorphisms to the risk of ischemic stroke. J Hypertens 38(8):1481–1487
Greco S, Fasanaro P, Castelvecchio S, D’Alessandra Y, Arcelli D, Di Donato M, Malavazos A, Capogrossi MC, Menicanti L, Martelli F (2012) MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes 61(6):1633–1641
Yan B, Zhang Y, Liang C, Liu B, Ding F, Wang Y, Zhu B, Zhao R, Yu XY, Li Y (2020) Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics 10(15):6728–6742
Ribeiro MF, Zhu H, Millard RW, Fan GC (2013) Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes 2(1):54–59
Johnson TK, Zhao L, Zhu D, Wang Y, Xiao Y, Oguljahan B, Zhao X, Kirlin WG, Yin L, Chilian WM et al (2019) Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am J Physiol Heart Circ Physiol 317(4):H765–H776
Peng X, Liang B, Wang H, Hou J, Yuan Q (2022) Hypoxia pretreatment improves the therapeutic potential of bone marrow mesenchymal stem cells in hindlimb ischemia via upregulation of NRG-1. Cell Tissue Res 388(1):105–116
Song S, Zhao Y, Fu T, Fan Y, Tang J, Wang X, Liu C, Chen X (2022) ELANE promotes M2 macrophage polarization by down-regulating PTEN and participates in the lung cancer progression. Immunol investig. https://doi.org/10.1080/08820139.2022.2115379
Huang D, Qiu H, Miao L, Guo L, Zhang X, Lin M, Li Z, Li F (2022) Cdc42 promotes thyroid cancer cell proliferation and migration and tumor-associated macrophage polarization through the PTEN/AKT pathway. J Biochem Mol Toxicol 36(8):e23115
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, He X, Zhong X, Li G, Chen Z, Li D (2020) Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol 13(1):156
Chen T, Liu Y, Li C, Xu C, Ding C, Chen J, Zhao J (2021) Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat Res Commun 28:100412
Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y, Liu L, Zhao W, Han Z, Kong D et al (2018) Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interf 10(36):30081–30091
Zhang K, Li Z (2020) Molecular imaging of therapeutic effect of mesenchymal stem cell-derived exosomes for hindlimb ischemia treatment. Methods Mol Biol 2150:213–225
Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A (2019) Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther 10(1):105
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B (2019) Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res 115(7):1205–1216
Sypecka M, Bzinkowska A, Sulejczak D, Dabrowski F, Sarnowska A (2022) Evaluation of the optimal manufacturing protocols and therapeutic properties of mesenchymal stem/stromal cells derived from wharton’s jelly. Int J Mol Sci 24(1):652
Han KH, Kim AK, Kim MH, Kim DH, Go HN, Kim DI (2016) Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol Int 40(1):27–35
Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC et al (2018) Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 8(22):6163–6177
Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamaziere JM et al (2010) Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 18(8):1545–1552
Chen L, Liu C, Sun D, Wang T, Zhao L, Chen W, Yuan M, Wang J, Lu W (2018) MicroRNA-133a impairs perfusion recovery after hindlimb ischemia in diabetic mice. Biosci Rep 38:4
Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T (2016) Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc 5:1
Clos-Sansalvador M, Garcia SG, Morón-Font M, Williams C, Reichardt NC, Falcón-Pérez JM, Bayes-Genis A, Roura S, Franquesa M, Monguió-Tortajada M, Borràs FE (2022) N-glycans in immortalized mesenchymal stromal cell-derived extracellular vesicles are critical for ev-cell interaction and functional activation of endothelial cells. Int J Mol Sci 23(17):9539
Wang Y, Smith W, Hao D, He B, Kong L (2019) M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol 70:459–466
Chang Q, Hao Y, Wang Y, Zhou Y, Zhuo H, Zhao G (2021) Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull 170:199–210
Li T, Gu J, Yang O, Wang J, Wang Y, Kong J (2020) Bone marrow mesenchymal stem cell-derived exosomal miRNA-29c decreases cardiac ischemia/reperfusion injury through inhibition of excessive autophagy via the PTEN/Akt/mTOR signaling pathway. Circ J 84(8):1304–1311
Wu L, Tian X, Zuo H, Zheng W, Li X, Yuan M, Tian X, Song H (2022) miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J Nanobiotechnol 20(1):196
Zare MA, Zare A, Azarpira N, Pakbaz S (2019) The protective effect of bone marrow-derived mesenchymal stem cells in liver ischemia/reperfusion injury via down-regulation of miR-370. Iran J Basic Med Sci 22(6):683–689
Acknowledgements
Not applicable.
Funding
This study was supported by Henan provincial joint construction project of medical science and technology of China (No. LHGJ20190857 to QY, No. LHGJ20190858 to XP).
Author information
Authors and Affiliations
Contributions
XP and QY: conceptualization, methodology,writing—original draft. JL, LR and BL: formal analysis. HW and JH: data curation. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared there is no conflict of interest to disclose.
Ethical approval and consent to participate
This study was permitted by the animal Ethics Committee of The Third Provincial People's Hospital of Henan Province.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11010_2023_4666_MOESM1_ESM.tif
Supplementary file1 (TIF 9392 KB) Supplemental Figure 1. (A) The expression of miR-34c in M0, M1, and M2 macrophages (n = 3, one-way ANOVA with Tukey’s post hoc test). The THP-1 cells were differentiated into M0 macrophages by incubating in 320 nM PMA for 24 h. THP-1 cells were differentiated into M1 macrophages by first being treated with 320 nM PMA for 6 h, followed by culturing by the addition of LPS. THP-1 cells were differentiated into M2 macrophages by first being treated with 320 nM PMA for 6 h, followed by cultured with IL-4 and IL-13 (20 ng/ml) for another 18 h. (B) The co-localization of miR-34c and the F4/80 antibody in Hyp-EV-treated mouse gastrocnemius muscle tissues.
11010_2023_4666_MOESM2_ESM.pdf
Supplementary file2 (PDF 449 KB) Supplemental Figure 2. Human BMMSCs transfected with LV-miR-34c inhibitor or LV-inhibitor-NC were treated with hypoxia or normoxia followed by the isolation of EVs. HUVECs were co-cultured with 30 μg/ml Nor-EVs or Hyp-EVs for 24 h. Cells were grouped as follows: control, Nor-EV, Hyp-EV, LV-in-NC-Hyp-EV, and LV-miR-34c in-Hyp-EV. (A) HUVEC viability was determined using the MTT cell proliferation and cytotoxicity assay kit (n = 3, one-way ANOVA with Tukey’s post hoc test). (B) Cell migration was observed using the Transwell assay (n = 3, one-way ANOVA with Tukey’s post hoc test). (C) Tube formation assay (n = 3, one-way ANOVA with Tukey’s post hoc test). *p < 0.05, **p<0.01, ***p < 0.001.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, X., Liu, J., Ren, L. et al. Extracellular vesicles derived from hypoxia-preconditioned bone marrow mesenchymal stem cells ameliorate lower limb ischemia by delivering miR-34c. Mol Cell Biochem 478, 1645–1658 (2023). https://doi.org/10.1007/s11010-023-04666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04666-7