Abstract
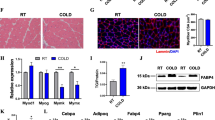
Cardiac function depends mainly on mitochondrial metabolism. Cold conditions increase the risk of cardiovascular diseases by increasing blood pressure. Adaptive thermogenesis leads to increased mitochondrial biogenesis and function in skeletal muscles and adipocytes. Here, we studied the effect of acute cold exposure on cardiac mitochondrial function and its regulation by sirtuins. Significant increase in mitochondrial DNA copy number as measured by the ratio between mitochondrial-coded COX-II and nuclear-coded cyclophilin A gene expression by qRT-PCR and increase in the expression of PGC-1α, a mitochondriogenic factor and its downstream target NRF-1 were observed on cold exposure. This was associated with an increase in the activity of SIRT-1, which is known to activate PGC-1α. Mitochondrial SIRT-3 was also upregulated. Increase in sirtuin activity was reflected in total protein acetylome, which decreased in cold-exposed cardiac tissue. An increase in mitochondrial MnSOD further indicated enhanced mitochondrial function. Further evidence for this was obtained from ex vivo studies of cardiac tissue treated with norepinephrine, which caused a significant increase in mitochondrial MnSOD and SIRT-3. SIRT-3 appears to mediate the regulation of MnSOD, as treatment with AGK-7, a SIRT-3 inhibitor reversed the norepinephrine-induced upregulation of MnSOD. It, therefore, appears that SIRT-3 activation in response to SIRT-1–PGC-1α activation contributes to the regulation of cardiac mitochondrial activity during acute cold exposure.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- BAT:
-
Brown adipose tissue
- NE:
-
Norepinephrine
- SNS:
-
Sympathetic nervous system
- Tfam:
-
Mitochondrial transcription factor A
- UCP-1:
-
Uncoupling Protein-1
- SOD:
-
Superoxide dismutase
References
Fares A (2013) Winter cardiovascular diseases phenomenon. N Am J Med Sci 5(4):266. https://doi.org/10.4103/1947-2714.110430
Liu C, Yavar Z, Sun Q (2015) Cardiovascular response to thermoregulatory challenges. Am J Physiology-Heart Circ Physiol 309(11):H1793–H1812. https://doi.org/10.1152/ajpheart.00199.2015
Bunker A, Wildenhain J, Vandenbergh A, Henschke N, Rocklöv J, Hajat S, Sauerborn R (2016) Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine 6:258–268. https://doi.org/10.1016/j.ebiom.2016.02.034
Ryti NR, Guo Y, Jaakkola JJ (2016) Global association of cold spells and adverse health effects: a systematic review and meta-analysis. Environ Health Perspect 124(1):12–22. https://doi.org/10.1289/ehp.1408104
Ryti NR, Mäkikyrö EM, Antikainen H, Hookana E, Junttila MJ, Ikäheimo TM, Kortelainen ML, Huikuri HV, Jaakkola JJ (2017) Risk of sudden cardiac death in relation to season-specific cold spells: a case–crossover study in Finland. BMJ Open 7(11):e017398. https://doi.org/10.1136/bmjopen-2017-017398
Song X, Wang S, Hu Y, Yue M, Zhang T, Liu Y, Tian J, Shang K (2017) Impact of ambient temperature on morbidity and mortality: an overview of reviews. Sci Total Environ 586:241–254. https://doi.org/10.1016/j.scitotenv.2017.01.212
Fregly MJ, Rossi F, Sun Z, Tümer N, Cade R, Hegland D, Yürekli M (1994) Effect of chronic treatment with prazosin and L-arginine on the elevation of blood pressure during cold exposure. Pharmacology 49(6):351–362. https://doi.org/10.1159/000139254
Sun Z, Cade R, Katovich MJ, Fregly MJ (1998) Body fluid distribution in rats with cold-induced hypertension. Physiol Behav 65(4–5):879–884. https://doi.org/10.1016/s0031-9384(98)00250-9
Sun Z, Cade R (2000) Cold-induced hypertension and diuresis. J Therm Biol 25(1–2):105–109. https://doi.org/10.1016/S0306-4565(99)00085-6
Fregly MJ, Kikta DC, Threatte RM, Torres JL, Barney CC (1989) Development of hypertension in rats during chronic exposure to cold. J Appl Physiol 66(2):741–749. https://doi.org/10.1152/jappl.1989.66.2.741
Sun Z, Cade R, Tatum C (2001) Central imidazoline and angiotensin II receptors in cardiovascular responses to chronic cold exposure in rats. J Therm Biol 26(4–5):513–518. https://doi.org/10.1016/S0306-4565(01)00071-7
Sun Z, Cade R, Morales C (2002) Role of central angiotensin II receptors in cold-induced hypertension. Am J Hypertens 15(1):85–92. https://doi.org/10.1016/s0895-7061(01)02230-0
Castellani CA, Longchamps RJ, Sun J, Guallar E, Arking DE (2020) Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 1(53):214–223. https://doi.org/10.1016/j.mito.2020.06.004
Longchamps RJ, Castellani CA, Yang SY, Newcomb CE, Sumpter JA, Lane J, Grove ML, Guallar E, Pankratz N, Taylor KD, Rotter JI (2020) Evaluation of mitochondrial DNA copy number estimation techniques. PLoS ONE 15(1):e0228166. https://doi.org/10.1371/journal.pone.0228166
Brown GC, Murphy MP, Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. https://doi.org/10.1042/bse0470069
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91(4):1309–1313. https://doi.org/10.1073/pnas.91.4.1309
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839. https://doi.org/10.1016/s0092-8674(00)81410-5
Gustafsson ÅB, Gottlieb RA (2008) Heart mitochondria: gates of life and death. Cardiovasc Res 77(2):334–343. https://doi.org/10.1093/cvr/cvm005
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295. https://doi.org/10.1146/annurev.pathol.4.110807.092250
Chang HC, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25(3):138–145. https://doi.org/10.1016/j.tem.2013.12.001
Kane AE, Sinclair DA (2018) Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res 123(7):868–885. https://doi.org/10.1161/circresaha.118.312498
Kupis W, Pałyga J, Tomal E, Niewiadomska E (2016) The role of sirtuins in cellular homeostasis. J Physiol Biochem 3:371–380. https://doi.org/10.1007/s13105-016-0492-6
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434(7029):113–118. https://doi.org/10.1038/nature03354
Yang W, Nagasawa K, Münch C, Xu Y, Satterstrom K, Jeong S, Hayes SD, Jedrychowski MP, Vyas FS, Zaganjor E, Guarani V (2016) Mitochondrial sirtuin network reveals dynamic SIRT3-dependent deacetylation in response to membrane depolarization. Cell 167(4):985–1000. https://doi.org/10.1016/j.cell.2016.10.016
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Investig 119(9):2758–2771. https://doi.org/10.1172/jci39162
Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA (2010) Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2(12):914. https://doi.org/10.18632/aging.100252
Sundaresan NR, Bindu S, Pillai VB, Samant S, Pan Y, Huang JY, Gupta M, Nagalingam RS, Wolfgeher D, Verdin E, Gupta MP (2015) SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol Cell Biol 36(5):678–692. https://doi.org/10.1128/mcb.00586-15
Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E (2013) The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY) 5(3):144. https://doi.org/10.18632/aging.100544
Tao R, Vassilopoulos A, Parisiadou L, Yan Y, Gius D (2014) Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid Redox Signal 20(10):1646–1654. https://doi.org/10.1089/ars.2013.5482
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL III, Goodyear LJ, Tong Q (2009) Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging (Albany NY) 1(9):771. https://doi.org/10.18632/aging.100075
Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y (2010) Sirtuin 3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE 5(7):e11707. https://doi.org/10.1371/journal.pone.0011707
Klingenberg M (1990) Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem Sci 15(3):108–112. https://doi.org/10.1016/0968-0004(90)90194-g
Cannon B, Nedergaard JAN (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359. https://doi.org/10.1152/physrev.00015.2003
Sellers EA, Scott JW, Thomas N (1954) Electrical activity of skeletal muscle of normal and acclimatized rats on exposure to cold. Am J Physiology-Legacy Content 177(3):372–376. https://doi.org/10.1152/ajplegacy.1954.177.3.372
Cottle WH, Carlson LD (1956) Regulation of Heat Production in Gold-Adapted Rats. Proc Soc Exp Biol Med 92(4):845–849. https://doi.org/10.3181/00379727-92-22632
Heroux OJSH, Hart JS, Depocas F (1956) Metabolism and muscle activity of anesthetized warm and cold acclimated rats on exposure to cold. J Appl Physiol 9(3):399–403. https://doi.org/10.1152/jappl.1956.9.3.399
Hart JS, Heroux O, Depocas F (1956) Cold acclimation and the electromyogram of unanesthetized rats. J Appl Physiol 9(3):404–408. https://doi.org/10.1152/jappl.1956.9.3.404
Hsieh AC, Carlson LD (1957) Role of adrenaline and noradrenaline in chemical regulation of heat production. Am J Physiology-Legacy Content 190(2):243–246. https://doi.org/10.1152/ajplegacy.1957.190.2.243
Hsieh ACL, Carlson LD, Gray G (1957) Role of the sympathetic nervous system in the control of chemical regulation of heat production. Am J Physiology-Legacy Content 190(2):247–251. https://doi.org/10.1152/ajplegacy.1957.190.2.247
Papanek PE, Wood CE, Fregly MJ (1991) Role of the sympathetic nervous system in cold-induced hypertension in rats. J Appl Physiol 71(1):300–306. https://doi.org/10.1152/jappl.1991.71.1.300
Chung N, Park J, Lim K (2017) The effects of exercise and cold exposure on mitochondrial biogenesis in skeletal muscle and white adipose tissue. J Exerc Nutr Biochem 21(2):39. https://doi.org/10.20463/jenb.2017.0020
Shi T, Wang F, Stieren E, Tong Q (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280(14):13560–13567. https://doi.org/10.1074/jbc.m414670200
Ikäheimo TM (2018) Cardiovascular diseases, cold exposure and exercise. Temperature 5(2):123–146
Engvall E, Perlmann P (1971) Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 8(9):871–874. https://doi.org/10.1016/0019-2791(71)90454-x
Rooney JP, Ryde IT, Sanders LH, Howlett EH, Colton MD, Germ KE, Mayer GD, Greenamyre JT, Meyer JN (2015) PCR based determination of mitochondrial DNA copy number in multiple species. Mitochondrial Regulation. Humana Press, New York, NY, pp 23–38
Edward E, Luk C, Culotta VC (2001) Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem 276(50):47556–47562. https://doi.org/10.1074/jbc.m108923200
Gamero-Sandemetrio E, Gómez-Pastor R, Matallana E (2013) Zymogram profiling of superoxide dismutase and catalase activities allows Saccharomyces and non-Saccharomyces species differentiation and correlates to their fermentation performance. Appl Microbiol Biotechnol 97(10):4563–4576. https://doi.org/10.1007/s00253-012-4672-1
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Watson SA, Scigliano M, Bardi I, Ascione R, Terracciano CM, Perbellini F (2017) Preparation of viable adult ventricular myocardial slices from large and small mammals. Nature Protoc 12(12):2623–2639. https://doi.org/10.1038/nprot.2017.139
Yang NC, Song TY, Chen MY, Hu ML (2011) Effects of 2-deoxyglucose and dehydroepiandrosterone on intracellular NAD+ level, SIRT1 activity and replicative lifespan of human Hs68 cells. Biogerontology 12(6):527–536. https://doi.org/10.1007/s10522-011-9342-7
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12(6):662–667. https://doi.org/10.1016/j.cmet.2010.11.015
Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y (2011) Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 12(6):534–541. https://doi.org/10.1038/embor.2011.65
Zhu Y, Park SH, Ozden O, Kim HS, Jiang H, Vassilopoulos A, Spitz DR, Gius D (2012) Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radical Biol Med 53(4):828–833. https://doi.org/10.1016/j.freeradbiomed.2012.06.020
Lowell BB, Spiegelman BM (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404(6778):652–660
Collins S (2012) β-Adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front Endocrinol 3(2):102. https://doi.org/10.3389/fendo.2011.00102
Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21(1):33–38. https://doi.org/10.1016/j.cmet.2014.12.009
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24(1):78–90. https://doi.org/10.1210/er.2002-0012
Popov LD (2020) Mitochondrial biogenesis: An update. J Cell Mol Med 24(9):4892–4899. https://doi.org/10.1111/jcmm.15194
Jeng JY, Yeh TS, Lee JW, Lin SH, Fong TH, Hsieh RH (2008) Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J Cell Biochem 103(2):347–357. https://doi.org/10.1002/jcb.21625
Butow RA, Bahassi EM (1999) Adaptive thermogenesis: orchestrating mitochondrial biogenesis. Curr Biol 9(20):R767–R769. https://doi.org/10.1016/s0960-9822(00)80008-1
Gao Y, Qimuge NR, Qin J, Cai R, Li X, Chu GY, Pang WJ, Yang GS (2018) Acute and chronic cold exposure differentially affects the browning of porcine white adipose tissue. Animal 12(7):1435–1441. https://doi.org/10.1017/s1751731117002981
Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA (2009) The role of PGC-1α on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol 297(1):C217–C225. https://doi.org/10.1152/ajpcell.00070.2009
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93(4):884S-S890. https://doi.org/10.3945/ajcn.110.001917
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104:12017–12022. https://doi.org/10.1073/pnas.0705070104
Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM (1999) Activation of PPARγ coactivator-1 through transcription factor docking. Science 286(5443):1368–1371. https://doi.org/10.1126/science.286.5443.1368
Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P (2006) GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab 3(6):429–438. https://doi.org/10.1016/j.cmet.2006.04.013
Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O’Malley BW, Auwerx J (2008) The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc Natl Acad Sci USA 105(44):17187–17192. https://doi.org/10.1073/pnas.0808207105
Park DR, Kim JS, Kim CK (2014) The effect of SIRT1 protein knock down on PGC-1α acetylation during skeletal muscle contraction. J Exerc Nutr Biochem 18(1):1. https://doi.org/10.5717/jenb.2014.18.1.1
Mulligan JD, Gonzalez AA, Stewart AM, Carey HV, Saupe KW (2007) Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J Physiol 580(2):677–684. https://doi.org/10.1113/jphysiol.2007.128652
Gerhart-Hines Z, Dominy JE Jr, Blättler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P (2011) The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol Cell 44(6):851–863. https://doi.org/10.1016/j.molcel.2011.12.005
Wei X, Jia R, Yang Z, Jiang J, Huang J, Yan J, Luo X (2020) NAD+/sirtuin metabolism is enhanced in response to cold-induced changes in lipid metabolism in mouse liver. FEBS Lett 594(11):1711–1725. https://doi.org/10.1002/1873-3468.13779
Perović A, Unić A, Dumić J (2014) Recreational scuba diving: negative or positive effects of oxidative and cardiovascular stress? Biochem Med 24(2):235–247. https://doi.org/10.11613/bm.2014.026
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27(24):8807–8814. https://doi.org/10.1128/mcb.01636-07
Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49(1):186–199. https://doi.org/10.1016/j.molcel.2012.10.024
Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E (2011) SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harbor symposia on quantitative biology, vol 76. Cold Spring Harbor Laboratory Press, Long Island, pp 267–277. https://doi.org/10.1101/sqb.2011.76.010850
Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK (2017) Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem 292(42):17312–17323. https://doi.org/10.1074/jbc.m117.778720
Holley AK, Clair DKS (2016) Manganese superoxide dismutase (MnSOD) and its importance in mitochondrial function and cancer. Redox-Active Therapeutics. Springer, Cham, pp 11–50. https://doi.org/10.1016/j.freeradbiomed.2012.03.009
Lu J, Zhang H, Chen X, Zou Y, Li J, Wang L, Wu M, Zang J, Yu Y, Zhuang W, Xia Q (2017) A small molecule activator of SIRT3 promotes deacetylation and activation of manganese superoxide dismutase. Free Radical Biol Med 112:287–297. https://doi.org/10.1016/j.freeradbiomed.2017.07.012
Murphy MP (2009) How mitochondria produce reactive oxygen species. Bioche J 417(1):1–13. https://doi.org/10.1042/BJ20081386
Yu T, Dohl J, Elenberg F, Chen Y, Deuster P (2019) Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts. J Cell Physiol 234(5):6371–6381. https://doi.org/10.1002/jcp.27370
Yoboue ED, Devin A (2012) Reactive oxygen species-mediated control of mitochondrial biogenesis. Int J Cell Biol. https://doi.org/10.1155/2012/403870
Belzer FO, Southard JH (1988) Principles of solid-organ preservation by cold storage. Transplantation 45(4):673–676. https://doi.org/10.1097/00007890-198804000-00001
Magni F, Panduri G, Paolocci N (1994) Hypothermia triggers iron-dependent lipoperoxidative damage in the isolated rat heart. Free Radical Biol Med 16(4):465–476. https://doi.org/10.1016/0891-5849(94)90124-4
Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF (2004) Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiology-Heart Circ Physiol 286(4):H1289–H1299. https://doi.org/10.1152/ajpheart.00811.2003
Zhang X, Liu H, Zhang D (2021) MnSOD serves as the central molecule in adaptive thermogenesis (MnSOD functions as a thermoreceptor). Adv Redox Res 3:100027. https://doi.org/10.1016/j.arres.2021.100027
Zhang X, Zhang D, Xiang L, Wang Q (2022) MnSOD functions as a thermoreceptor activated by low temperature. J Inorgan Biochem 1(229):111745. https://doi.org/10.1016/j.jinorgbio.2022.111745
Funding
Mithra. S. Mohan was supported by the Kerala State Council for Science, Technology and Environment in the form of research fellowship. Saja. K was supported by the University Grants Commission—Basic Scientific Research in the form of start-up grant—and the Department of Science and Technology—Science Engineering Research Board in the form of Young investigator program. Aswani. S. S and Aparna.N. S were supported by Joint CSIR-UGC fellowship under UGC scheme.
Author information
Authors and Affiliations
Contributions
Mithra. S. Mohan, P. R. Sudhakaran and Saja. K conceptualized the study and planned the experiments and discussed the results. Mithra. S. Mohan wrote the draft of the manuscript. Saja. K, P. R. Sudhakaran and P. T. Boban reviewed and edited the manuscript. Mithra. S. Mohan, Aswani. S. S. and Aparna N. S. carried out the experiments. All authors participated in the design of the study and interpretation and analysis of the data and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Experiments in this research were reviewed and approved by the animal ethical committee, University of Kerala (IAEC-KU-12b/2014-15).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohan, M.S., Aswani, S.S., Aparna, N.S. et al. Effect of acute cold exposure on cardiac mitochondrial function: role of sirtuins. Mol Cell Biochem 478, 2257–2270 (2023). https://doi.org/10.1007/s11010-022-04656-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04656-1