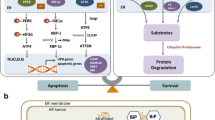
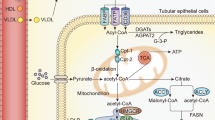
Abstract
Kidney disease is the 6th fastest-growing cause of death and a serious global health concern that urges effective therapeutic options. The inflammatory response is an initial reaction from immune and parenchymal cells in kidney diseases. Toll-like receptors (TLR) 2 and 4 are highly expressed by various kidney cells and respond to ‘signaling danger’ proteins, such as high mobility group box binding protein 1 (HMGB1) and prompt the progression of kidney disease by releasing inflammatory mediators. Burgeoning reports suggest that both SGLT2 and ER stress elevates TLR2/4 signaling via different axis. Moreover, SGLT2 signaling aggravates inflammation under the disease condition by promoting the NLR family pyrin domain-containing three inflammasomes and ER stress. Intriguingly, TLR2/4 downstream adaptors activate ER stress regulators. The above-discussed interactions imply that TLR2/4 does more than immune response during kidney disease. Here, we discuss in detail evidence of the roles and regulation of TLR2/4 in the context of a relationship between ER stress and SGLT2. Also, we highlighted different preclinical studies of SGLT2 inhibitors against TLR2/4 signaling in various kidney diseases. Moreover, we discuss the observational and interventional evidence about the relation between TLR2/4, ER stress, and SGLT2, which may represent the TLR2/4 as a potential therapeutic target for kidney disease.



Similar content being viewed by others
Data availability
Not applicable.
References
Carney EF (2020) The impact of chronic kidney disease on global health. Nat Rev Nephrol 16(5):251–251. https://doi.org/10.1038/s41581-020-0268-7
Luyckx VA, Tonelli M, Stanifer JW (2018) The global burden of kidney disease and the sustainable development goals. Bull World Health Organ 96(6):414–422. https://doi.org/10.2471/BLT.17.206441
Gaudry S, Hajage D, Benichou N et al (2020) Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. The Lancet 395(10235):1506–1515. https://doi.org/10.1016/S0140-6736(20)30531-6
Komada T, Muruve DA (2019) The role of inflammasomes in kidney disease. Nat Rev Nephrol 15(8):501–520. https://doi.org/10.1038/s41581-019-0158-z
Aly RH, Ahmed AE, Hozayen WG et al (2020) Patterns of toll-like receptor expressions and inflammatory cytokine levels and their implications in the progress of insulin resistance and diabetic nephropathy in type 2 diabetic patients. Front Physiol 11:1723
Anders HJ (2010) Toll-like receptors and danger signaling in kidney injury. J Am Soc Nephrol 21(8):1270–1274. https://doi.org/10.1681/asn.2010030233
Patole PS, Schubert S, Hildinger K et al (2005) Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int 68(6):2582–2587. https://doi.org/10.1111/j.1523-1755.2005.00729.x
Ma J, Chadban SJ, Zhao CY et al (2014) TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE 9(5):e97985. https://doi.org/10.1371/journal.pone.0097985
Ma K, Li J, Wang X et al (2018) TLR4(+)CXCR4(+) plasma cells drive nephritis development in systemic lupus erythematosus. Ann Rheum Dis 77(10):1498–1506. https://doi.org/10.1136/annrheumdis-2018-213615
Fan Y, Xiao W, Lee K et al (2017) Inhibition of reticulon-1A-mediated endoplasmic reticulum stress in early AKI attenuates renal fibrosis development. J Am Soc Nephrol 28(7):2007. https://doi.org/10.1681/ASN.2016091001
Gómez-Sierra T, Bellido B, Reyes-Fermín LM et al (2021) Regulation of endoplasmic reticulum stress in models of kidney disease. Adv Redox Res 3:100010. https://doi.org/10.1016/j.arres.2021.100010
Shu S, Zhu J, Liu Z et al (2018) Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 37:269–280. https://doi.org/10.1016/j.ebiom.2018.10.006
Tam AB, Mercado EL, Hoffmann A et al (2012) ER Stress Activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS ONE 7(10):e45078. https://doi.org/10.1371/journal.pone.0045078
Wang Q-L, Xing W, Yu C et al (2021) ROCK1 regulates sepsis-induced acute kidney injury via TLR2-mediated endoplasmic reticulum stress/pyroptosis axis. Mol Immunol 138:99–109. https://doi.org/10.1016/j.molimm.2021.07.022
Ashrafi Jigheh Z, Ghorbani Haghjo A, Argani H et al (2019) Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran J Basic Med Sci 22(4):384–390. https://doi.org/10.22038/ijbms.2019.31788.7651
Kuno A, Kimura Y, Mizuno M et al (2020) Empagliflozin attenuates acute kidney injury after myocardial infarction in diabetic rats. Sci Rep 10(1):7238. https://doi.org/10.1038/s41598-020-64380-y
Chen J, Mohler ER, Xie D et al (2016) Traditional and non-traditional risk factors for incident peripheral arterial disease among patients with chronic kidney disease. Nephrol Dial Transplant 31(7):1145–1151. https://doi.org/10.1093/ndt/gfv418
Chen Q, Guan X, Zuo X et al (2016) The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm Sin B 6(3):183–188. https://doi.org/10.1016/j.apsb.2016.02.004
Mima A (2022) Mitochondria-targeted drugs for diabetic kidney disease. Heliyon 8(2):e08878. https://doi.org/10.1016/j.heliyon.2022.e08878
Sharma N, Anders HJ, Gaikwad AB (2019) Fiend and friend in the renin angiotensin system: an insight on acute kidney injury. Biomed Pharmacother 110:764–774. https://doi.org/10.1016/j.biopha.2018.12.018
Kazancioğlu R (2013) Risk factors for chronic kidney disease: an update. Kidney Int Suppl 3(4):368–371. https://doi.org/10.1038/kisup.2013.79
Premkumar V, Dey M, Dorn R et al (2010) MyD88-dependent and independent pathways of toll-like receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem Biol 10:3–3. https://doi.org/10.1186/1472-6769-10-3
Jain S, Plenter R, Nydam T et al (2021) Deletion of TLR4 reduces apoptosis and improves histology in a murine kidney transplant model. Sci Rep 11(1):16182. https://doi.org/10.1038/s41598-021-95504-7
Krüger B, Krick S, Dhillon N et al (2009) Donor toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci 106(9):3390. https://doi.org/10.1073/pnas.0810169106
Jain S, Keys D, Ljubanovic D et al (2015) Protection against cold storage-induced renal tubular cell apoptosis. Transplantation 99(11):2311–2316. https://doi.org/10.1097/tp.0000000000000774
Urbschat A, Baer P, Zacharowski K et al (2018) Systemic TLR2 antibody application in renal ischaemia and reperfusion injury decreases AKT phosphorylation and increases apoptosis in the mouse kidney. Basic Clin Pharmacol Toxicol 122(2):223–232. https://doi.org/10.1111/bcpt.12896
Yuan S, Liu X, Zhu X et al (2018) The role of TLR4 on PGC-1α-mediated oxidative stress in tubular cell in diabetic kidney disease. Oxid Med Cell Longev 2018:6296802. https://doi.org/10.1155/2018/6296802
Bell CW, Jiang W et al (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291(6):C1318–C1325. https://doi.org/10.1152/ajpcell.00616.2005
Huang Z, Zhong Z, Zhang L et al (2015) Down-regulation of HMGB1 expression by shRNA constructs inhibits the bioactivity of urothelial carcinoma cell lines via the NF-κB pathway. Sci Rep 5(1):12807. https://doi.org/10.1038/srep12807
Zhao X, Kuja-Panula J, Fau-Rouhiainen A, Rouhiainen A, Fau-Chen Y-C et al (2011) High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. J Biol Chem 286(26):23200–23213. https://doi.org/10.1074/jbc.M111.223834
Evankovich J, Cho SW, Zhang R et al (2010) High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem 285(51):39888–39897. https://doi.org/10.1074/jbc.M110.128348
Yang H, Antoine DJ, Andersson U et al (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93(6):865–873. https://doi.org/10.1189/jlb.1212662
Zhou X, Lin N, Zhang M et al (2020) Circulating soluble receptor for advanced glycation end products and other factors in type 2 diabetes patients with colorectal cancer. BMC Endocr Disord 20(1):170. https://doi.org/10.1186/s12902-020-00647-9
Zhou Y, Fau-Zhou LS et al (2021) Research on the relationship between RAGE and its ligand HMGB1, and prognosis and pathogenesis of gastric cancer with diabetes mellitus. Eur Rev Med Pharmacol Sci 25(3):1339–1350. https://doi.org/10.26355/eurrev_202102_24841
Chhipa AS, Borse SP, Baksi R et al (2019) Targeting receptors of advanced glycation end products (RAGE): preventing diabetes induced cancer and diabetic complications. Pathol Res Pract 215(11):152643. https://doi.org/10.1016/j.prp.2019.152643
Chen R-C, Yi P-P, Zhou R-R et al (2014) The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines. Mol Cell Biochem 390(1):271–280. https://doi.org/10.1007/s11010-014-1978-6
Fahmueller YN, Nagel D, Hoffmann R-T et al (2013) Immunogenic cell death biomarkers HMGB1, RAGE, and DNAse indicate response to radioembolization therapy and prognosis in colorectal cancer patients. Int J Cancer 132(10):2349–2358. https://doi.org/10.1002/ijc.27894
Wu H, Ma J, Wang P et al (2010) HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21(11):1878. https://doi.org/10.1681/ASN.2009101048
Agalave NM, Svensson CI (2015) Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol Med (Cambridge) 20(1):569–578. https://doi.org/10.2119/molmed.2014.00176
Oh SM, Park G, Lee SH et al (2017) Assessing the recovery from prerenal and renal acute kidney injury after treatment with single herbal medicine via activity of the biomarkers HMGB1, NGAL and KIM-1 in kidney proximal tubular cells treated by cisplatin with different doses and exposure times. BMC Complement Altern Med 17(1):544. https://doi.org/10.1186/s12906-017-2055-y
Bruchfeld A, Qureshi AR, Lindholm B et al (2008) High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med 14(3–4):109–115. https://doi.org/10.2119/2007-00107
Oh H, Choi A, Seo N et al (2021) Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on post-contrast acute kidney injury. Sci Rep 11(1):15625. https://doi.org/10.1038/s41598-021-94928-5
Sun N, Wang H, Wang L (2016) Protective effects of ghrelin against oxidative stress, inducible nitric oxide synthase and inflammation in a mouse model of myocardial ischemia/reperfusion injury via the HMGB1 and TLR4/NF-κB pathway. Mol Med Rep 14(3):2764–2770. https://doi.org/10.3892/mmr.2016.5535
Zhang H, Zhang R, Chen J et al (2017) High mobility group box1 inhibitor glycyrrhizic acid attenuates kidney injury in streptozotocin-induced diabetic rats. Kidney Blood Press Res 42(5):894–904. https://doi.org/10.1159/000485045
Seo MS, Kim HJ, Kim H et al (2019) Ethyl pyruvate directly attenuates active secretion of HMGB1 in proximal tubular cells via induction of heme oxygenase-1. J. Clin. Med. 8(5):629. https://doi.org/10.3390/jcm8050629
Bonaldi T, Talamo F, Scaffidi P et al (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22(20):5551–5560. https://doi.org/10.1093/emboj/cdg516
Ruan Y, Wang L, Zhao Y et al (2014) Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int 86(3):525–537. https://doi.org/10.1038/ki.2014.80
Sankrityayan H, Oza MJ, Kulkarni YA et al (2019) ER stress response mediates diabetic microvascular complications. Drug Discov Today 24(12):2247–2257. https://doi.org/10.1016/j.drudis.2019.08.003
Cybulsky AV (2017) Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13(11):681–696. https://doi.org/10.1038/nrneph.2017.129
Bartoszewska S, Collawn JF (2020) Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell Mol Biol Lett 25(1):18. https://doi.org/10.1186/s11658-020-00212-1
Cao Y, Trillo-Tinoco J, Sierra RA et al (2019) ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat Commun 10(1):1280. https://doi.org/10.1038/s41467-019-09263-1
Wei S, Gao Y, Dai X et al (2018) SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol-Renal Physiol 316(1):F20–F31. https://doi.org/10.1152/ajprenal.00119.2018
González-Guerrero C, Ocaña-Salceda C, Berzal S et al (2013) Calcineurin inhibitors recruit protein kinases JAK2 and JNK, TLR signaling and the UPR to activate NF-κB-mediated inflammatory responses in kidney tubular cells. Toxicol Appl Pharmacol 272(3):825–841. https://doi.org/10.1016/j.taap.2013.08.011
Hu H, Tian M, Ding C et al (2019) The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 9:3083
Mostafa RG, El Abd AE, Fouda EA, Taha FR, Elzorkany KM (2020) A pilot study on gene expression of endoplasmic reticulum unfolded protein response in chronic kidney disease. Biochem Biophys Rep 24:100829. https://doi.org/10.1016/j.bbrep.2020.100829
Liu S-H, Wu C-T, Huang K-H et al (2016) C/EBP homologous protein (CHOP) deficiency ameliorates renal fibrosis in unilateral ureteral obstructive kidney disease. Oncotarget 7:16
Zhang M, Guo Y, Fu H et al (2015) Chop deficiency prevents UUO-induced renal fibrosis by attenuating fibrotic signals originated from Hmgb1/TLR4/NFκB/IL-1β signaling. Cell Death Dis 6(8):e1847. https://doi.org/10.1038/cddis.2015.206
Shen X, Weng C, Wang Y et al (2020) Lipopolysaccharide-induced podocyte injury is regulated by calcineurin/NFAT and TLR4/MyD88/NF-κB signaling pathways through angiopoietin-like protein 4. Genes Dis. https://doi.org/10.1016/j.gendis.2020.07.005
Yang CC, Yao CA, Yang JC et al (2014) Sialic acid rescues repurified lipopolysaccharide-induced acute renal failure via inhibiting TLR4/PKC/gp91-mediated endoplasmic reticulum stress, apoptosis, autophagy, and pyroptosis signaling. Toxicol Sci 141(1):155–165. https://doi.org/10.1093/toxsci/kfu121
Mima A, Hiraoka-Yamomoto J, Li Q, Kitada M, Li C, Geraldes P, Matsumoto M, Mizutani K, Park K, Cahill C, Nishikawa SI (2012) Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 61(11):2967–2979. https://doi.org/10.2337/db11-1824
Mima A, Ohshiro Y, Kitada M, Matsumoto M et al (2011) Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int 79(8):883–96. https://doi.org/10.1038/ki.2010.526
Serrero M, Planès R, Bahraoui E (2017) PKC-δ isoform plays a crucial role in Tat-TLR4 signalling pathway to activate NF-κB and CXCL8 production. Sci Rep 7(1):2384. https://doi.org/10.1038/s41598-017-02468-8
Wang N, Mao L, Yang L et al (2017) Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 8(22):36449–36461. https://doi.org/10.18632/oncotarget.16860
Wang XX, Levi J, Luo Y et al (2017) SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice*. J Biol Chem 292(13):5335–5348. https://doi.org/10.1074/jbc.M117.779520
Mima A, Kitada M, Geraldes P, Li Q, Matsumoto M, Mizutani K, Qi W, Li C, Leitges M, Rask-Madsen C, King GL (2012) Glomerular VEGF resistance induced by PKCδ/SHP-1 activation and contribution to diabetic nephropathy. FASEB J 26(7):2963–2974. https://doi.org/10.1096/fj.11-202994
Mima A, Yasuzawa T, King GL et al (2018) Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Biol 8(4):664–670. https://doi.org/10.1002/2211-5463.12400
Möller-Hackbarth K, Dabaghie D, Charrin E et al (2021) Retinoic acid receptor responder1 promotes development of glomerular diseases via the nuclear factor-κB signaling pathway. Kidney Int 100(4):809–823. https://doi.org/10.1016/j.kint.2021.05.036
Shen J, Dai Z, Li Y et al (2022) TLR9 regulates NLRP3 inflammasome activation via the NF-kB signaling pathway in diabetic nephropathy. Diabetol Metab Syndr 14(1):26. https://doi.org/10.1186/s13098-021-00780-y
Ferrè S, Deng Y, Huen SC et al (2019) Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation. Kidney Int 96(6):1359–1373. https://doi.org/10.1016/j.kint.2019.06.023
Woo CW, Kutzler L, Kimball SR et al (2012) Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol 14(2):192–200. https://doi.org/10.1038/ncb2408
Lai H-J, Zhan Y-Q, Qiu Y-X et al (2021) HMGB1 signaling-regulated endoplasmic reticulum stress mediates intestinal ischemia/reperfusion-induced acute renal damage. Surgery 170(1):239–248. https://doi.org/10.1016/j.surg.2021.01.042
Fohlen B, Tavernier Q, Huynh T-M et al (2018) Real-time and non-invasive monitoring of the activation of the IRE1α-XBP1 pathway in individuals with hemodynamic impairment. EBioMedicine 27:284–292. https://doi.org/10.1016/j.ebiom.2017.12.023
Mami I, Bouvier N, El Karoui K et al (2016) Angiogenin mediates cell-autonomous translational control under endoplasmic reticulum stress and attenuates kidney injury. J Am Soc Nephrol 27(3):863. https://doi.org/10.1681/ASN.2015020196
Tavernier Q, Mami I, Rabant M et al (2017) Urinary angiogenin reflects the magnitude of kidney injury at the infrahistologic level. J Am Soc Nephrol 28(2):678–690. https://doi.org/10.1681/asn.2016020218
Schaafhausen MK, Yang WJ, Centanin L et al (2013) Tumor angiogenesis is caused by single melanoma cells in a manner dependent on reactive oxygen species and NF-κB. J Cell Sci 126(Pt 17):3862–3872. https://doi.org/10.1242/jcs.125021
van Bommel EJM, Muskiet MHA, Tonneijck L et al (2017) SGLT2 inhibition in the diabetic kidney—from mechanisms to clinical outcome. Clin J Am Soc Nephrol 12(4):700. https://doi.org/10.2215/CJN.06080616
Mima A (2018) Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabetes Complic 32(7):720–725. https://doi.org/10.1016/j.jdiacomp.2018.04.011
Mudaliar H, Pollock C, Komala MG et al (2013) The role of toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol 305(2):F143-154. https://doi.org/10.1152/ajprenal.00398.2012
Yao D, Wang S, Wang M et al (2018) Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-κB signaling pathway. Mol Med Rep 18(4):3625–3630. https://doi.org/10.3892/mmr.2018.9393
Panchapakesan U, Pegg K, Gross S et al (2013) Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS ONE 8(2):e54442. https://doi.org/10.1371/journal.pone.0054442
Kabel AM, Salama SA (2021) Effect of taxifolin/dapagliflozin combination on colistin-induced nephrotoxicity in rats. Hum Exp Toxicol 40(10):1767–1780. https://doi.org/10.1177/09603271211010906
Kimura Y, Kuno A, Tanno M et al (2019) Canagliflozin, a sodium–glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J Diabetes Investig 10(4):933–946. https://doi.org/10.1111/jdi.13009
Ashrafi Jigheh Z, Ghorbani Haghjo A (2020) Empagliflozin attenuates renal and urinary markers of tubular epithelial cell injury in streptozotocin-induced diabetic rats. Indian J Clin Biochem 35(1):109–114. https://doi.org/10.1007/s12291-018-0790-6
Kajiwara K, Sawa Y (2021) Overexpression of SGLT2 in the kidney of a P. gingivalis LPS-induced diabetic nephropathy mouse model. BMC Nephrol 22(1):287. https://doi.org/10.1186/s12882-021-02506-8
Kim SR, Lee SG (2020) SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 11(1):2127. https://doi.org/10.1038/s41467-020-15983-6
Chen X, Guo X, Ge Q et al (2019) ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis. Oxid Med Cell Longev 2019:3462530. https://doi.org/10.1155/2019/3462530
Shibusawa R, Yamada E, Okada S et al (2019) Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci Rep 9(1):9887. https://doi.org/10.1038/s41598-019-46402-6
Fernandes-Alnemri T, Kang S, Anderson C et al (2013) Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol (Baltimore) 191(8):3995–3999. https://doi.org/10.4049/jimmunol.1301681
Delanaye P, Scheen AJ (2021) Epidemiology of acute kidney injury adverse events with SGLT2 inhibitors: a meta-analysis of observational cohort studies. Diabetes Epidemiol Manag 3:100021. https://doi.org/10.1016/j.deman.2021.100021
Rampersad C, Kraut E, Whitlock RH et al (2020) Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: a retrospective cohort study. Am J Kidney Dis 76(4):471–479. https://doi.org/10.1053/j.ajkd.2020.03.019
Shih J-Y, Lin Y-W, Fisch S et al (2020) Dapagliflozin suppresses ER stress and improves subclinical myocardial function in diabetes: from bedside to bench. Diabetes 70(1):262–267. https://doi.org/10.2337/db20-0840
Acknowledgements
ABG sincerely acknowledges the financial support provided by the Birla Institute of Technology and Science, Pilani, Pilani Campus, for carrying out this work. HJA was supported by the Deutsche Forschungsgemeinschaft (AN372/14-4 and 30-1).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
VS: Literature research, Writing—Original draft preparation, Review. AK: Writing—Review & Editing. H-JA: Participated in designing the manuscript, edited, and prepared it for submission. ABG: Conceptualization, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shelke, V., Kale, A., Anders, HJ. et al. Toll-like receptors 2 and 4 stress signaling and sodium-glucose cotransporter-2 in kidney disease. Mol Cell Biochem 478, 1987–1998 (2023). https://doi.org/10.1007/s11010-022-04652-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04652-5