Abstract
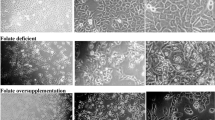
Folate is a vital vitamin involved in one-carbon metabolism and any changes in folate status may lead to epigenetic alterations. It is already known that stages and liver cancer progression are negatively correlated with folate levels. Nevertheless, mechanisms involved in folate deficiency in HCC (Hepatocellular carcinoma) are still not completely understood. So, this study tests the hypothesis that due to the increased demand for ER (endoplasmic reticulum) proteins, folate deficiency might lead to the induction of UPR (unfolded protein response), which is further correlated with HCC outcomes. HCC cells were cultured in both folate normal (FN) and folate deficient (FD) conditions and the expression of genes of ER stress pathway was investigated. The results demonstrated activation of UPR via induction of PERK, ATF4, and LAMP3. Besides this, FD reduced the migratory capacity and the invasiveness of HCC cells along with the reduction in mesenchymal markers like vimentin but increased apoptosis. Treatment with GSK2606414 (PERK inhibitor) decreased the FD induced expression of PERK, ATF4, and LAMP3 in FD cells. Also, GSK2606414 was found to increase apoptotic cell death and to further reduce the cancer hallmarks selectively in FD cells but not in FN cells. Altogether, our data suggest that targeting the ER stress pathway along with folate deficiency may provide a more promising elimination of the metastatic potential of HCC cells contributing to more effective therapeutic agents.
Graphical abstract









Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- PERK:
-
Protein kinase R (PKR)-like endoplasmic reticulum kinase
- ATF4:
-
Activating transcription factor 4
- LAMP3:
-
Lysosome-associated membrane glycoprotein 3
References
Heindryckx F, Gerwins P (2015) Targeting the tumor stroma in hepatocellular carcinoma. World J Hepatol 7(2):165–176. https://doi.org/10.4254/wjh.v7.i2.165
Tian Y, Wong VWS, Chan HLY, Cheng ASL (2013) Epigenetic regulation of hepatocellular carcinoma in non-alcoholic fatty liver disease. Semin Cancer Biol 23:471–482. https://doi.org/10.1016/j.semcancer.2013.08.010
Zhang W, Shu XO, Li H, Yang G, Cai H, Ji B et al (2012) Vitamin intake and liver cancer risk: a report from two cohort studies in China. J Natl Cancer Inst 104:1173–1181. https://doi.org/10.1093/jnci/djs277
Crider KS, Yang TP, Berry RJ, Bailey LB (2012) Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 3:21–38. https://doi.org/10.3945/an.111.000992
Varela-Moreiras G, Selhub J (1990) Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr 122:986–991. https://doi.org/10.1093/jn/122.4.986
Kuo CS, Lin CY, Wu MY, Lu CL, Huang RF (2008) Relationship between folate status and tumour progression in patients with hepatocellular carcinoma. Br J Nutr 100:596–602. https://doi.org/10.1017/S0007114508911557
van der Heiden MG, Cantley LC, Thompson CB (2009) Understanding the warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–33. https://doi.org/10.1126/science.1160809
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789. https://doi.org/10.1146/annurev.biochem.73.011303.074134
Fernandez PM, Tabbara SO, Jacobs LK, Manning FCR, Tsangaris TN, Schwartz AM et al (2000) Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat 59:15–26. https://doi.org/10.1023/a:1006332011207
Feng YX, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JHL, Proia TA et al (2014) Epithelial-to-mesenchymal transition activates PERK-eIF2α and sensitizes cells to endoplasmic reticulum stress. Cancer Discov 4:702–715. https://doi.org/10.1158/2159-8290.CD-13-0945
Li Y, Du W, Han J, Ge J (2017) LAMP3 promotes the invasion of Osteosarcoma cells via SPP1 signaling. Mol Med Rep 16:5947–5953. https://doi.org/10.3892/mmr.2017.7349
Mujcic H, Rzymski T, Rouschop KMA, Koritzinsky M, Milani M, Harris AL et al (2009) Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol 92:450–459. https://doi.org/10.1016/j.radonc.2009.08.017
Ji C, Kaplowitz N (2004) Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol 10:1699–1708. https://doi.org/10.1055/s-2007-991513
Mahameed M, Wilhelm T, Darawshi O, Obiedat A, Tommy WS, Chintha C et al (2019) The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell Death Dis 10:300. https://doi.org/10.1038/s41419-019-1523-3
Zhang M, Wang B, Takayama T, Shi X, Roenneburg DA, Kent KC et al (2015) Blocking bromo- and extra-terminal bromodomains mitigates intimal hyperplasia in rat carotid arteries: role of epigenetic reader in vascular disease. J Am Coll Surg 11:1650–1661. https://doi.org/10.1016/j.ebiom.2015.09.045
Liu J, Ward RL (2010) Folate and one-carbon metabolism and its impact on aberrant DNA methylation in cancer. Adv Genet 71:79–121. https://doi.org/10.1016/B978-0-12-380864-6.00004-3
Tripathi M, Zhang CW, Singh BK, Sinha RA, Moe KT, Desilva DA et al (2016) Hyperhomocysteinemia causes ER stress and impaired autophagy that is reversed by vitamin B supplementation. Cell Death Dis 7:e2513. https://doi.org/10.1038/cddis.2016.374
Kao TT, Chu CY, Lee GH, Hsiao TH, Cheng NW, Chang NS et al (2014) Folate deficiency-induced oxidative stress contributes to neuropathy in young and aged zebrafish: implication in neural tube defects and Alzheimer’s diseases. Neurobiol Dis 71:234–244. https://doi.org/10.1016/j.nbd.2014.08.004
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M et al (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633. https://doi.org/10.1016/s1097-2765(03)00105-9
Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC et al (2013) Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res 15:R2. https://doi.org/10.1186/bcr3373
Feo F, Pascale RM, Simile MM, De Miglio MR, Muroni MR, Calvisi D (2000) Genetic alterations in liver carcinogenesis: implications for new preventive and therapeutic strategies. Crit Rev Oncog 11:19–62
Limia CM, Sauzay C, Urra H, Hetz C, Chevet E, Avril T (2019) Emerging roles of the endoplasmic reticulum associated unfolded protein response in cancer cell migration and invasion. Cancers (Basel) 11:631. https://doi.org/10.3390/cancers11050631
Farias N, Ho N, Butler S, Delaney L, Morrison J, Shahrzad S et al (2015) The effects of folic acid on global DNA methylation and colonosphere formation in colon cancer cell lines. J Nutr Biochem 26(8):818–826. https://doi.org/10.1016/j.jnutbio.2015.02.002
Liang Y, Cao D, Li Y, Liu Z, Wu J (2020) MicroRNA-302a is involved in folate deficiency-induced apoptosis through the AKT-FOXO1-BIM pathway in mouse embryonic stem cells. Nutr Metab 17:103. https://doi.org/10.1186/s12986-020-00530-3
Mounir Z, Krishnamoorthy JL, Wang S, Papadopoulou B, Campbell S, Muller WJ et al (2011) Akt determines cell fate through inhibition of the PERK-eIF2α phosphorylation pathway. Sci Signal 4:ra2. https://doi.org/10.1126/scisignal.2001630
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Hu P, Han Z, Couvillon AD, Exton JH (2004) Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem 279:49420–49429. https://doi.org/10.1038/sj.embor.7400779
Chunhua L, Donglan L, Xiuqiong F, Lihua Z, Qin F, Yawei L et al (2013) Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J Nutr Biochem 24:1766–1775. https://doi.org/10.1016/j.jnutbio.2013.03.006
Acknowledgements
We are so grateful to Mr. Ravjit Singh (Senior Lab Technician) for his help in flow cytometry analysis.
Funding
We are thankful to DST-FIST for the sponsorship of the flow cytometry facility.
Author information
Authors and Affiliations
Contributions
HG: Writing-original draft, Methodology, Data curation. RS: Supervision, Investigation, Visualization. DL: Software, Validation. JK: Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goyal, H., Sharma, R., Lamba, D. et al. Folic acid depletion along with inhibition of the PERK arm of endoplasmic reticulum stress pathway promotes a less aggressive phenotype of hepatocellular carcinoma cells. Mol Cell Biochem 478, 2057–2068 (2023). https://doi.org/10.1007/s11010-022-04651-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04651-6